Solutions
keyboard_arrow_downServices
keyboard_arrow_downSupport & Resources
keyboard_arrow_downCompany
keyboard_arrow_downContact
The RIQAS Pre-eclampsia EQA Programme is designed to monitor a laboratories ability to correctly determine the outcome of the following tests, which are useful for screening, diagnosing, predicting and monitoring placenta related disorders; PAPP-A, PlGF, sFlt-1 and sFlt-1/PlGF ratio. Ensuring accuracy in Pre-eclampsia testing, helping to improve patient care and outcomes
Lyophilised material for enhanced stability

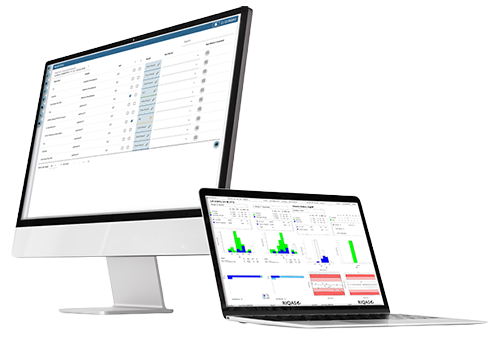
Submit results and view reports online via RIQAS.Net
World's largest EQA scheme ensuring peer groups are maximised
Monthly reporting for earlier identification of test system errors
Cycle Starts - July 2026
Not Accredited
Get in touch to discover more
To find out more about RIQAS EQA or to get in touch with your local Randox Representative, enquire now.
| Description | Frequency | Size | Cat No | |
|---|---|---|---|---|
| Pre-eclampsia | Monthly | 12 x 1ml | RQ9207 |
Parameters
- PAPP-A
- sFlt-1
- sFlt-1/PlGF ratio
- PlGF
Related RIQAS EQA Programmes
Maternal Screening
General Clinical Chemistry
Specific Proteins