Solutions
keyboard_arrow_downServices
keyboard_arrow_downSupport & Resources
keyboard_arrow_downCompany
keyboard_arrow_downContact
Versatile Test Menu
Leading multiplex infectious disease test menu comprising respiratory, genitourinary and hospital acquired infections.
Flexibility
Compatible with various samples (urine, nasal/throat swabs, sputum), the multiplex technology detects multiple targets simultaneously with both High-Plex and Low-Plex testing.
Ideal for Point of Care
Preloaded with all reagents, the sealed Vivalytic cartridge minimizes prep time and stores easily at room temperature — no refrigeration needed.
Current Testing Portfolio
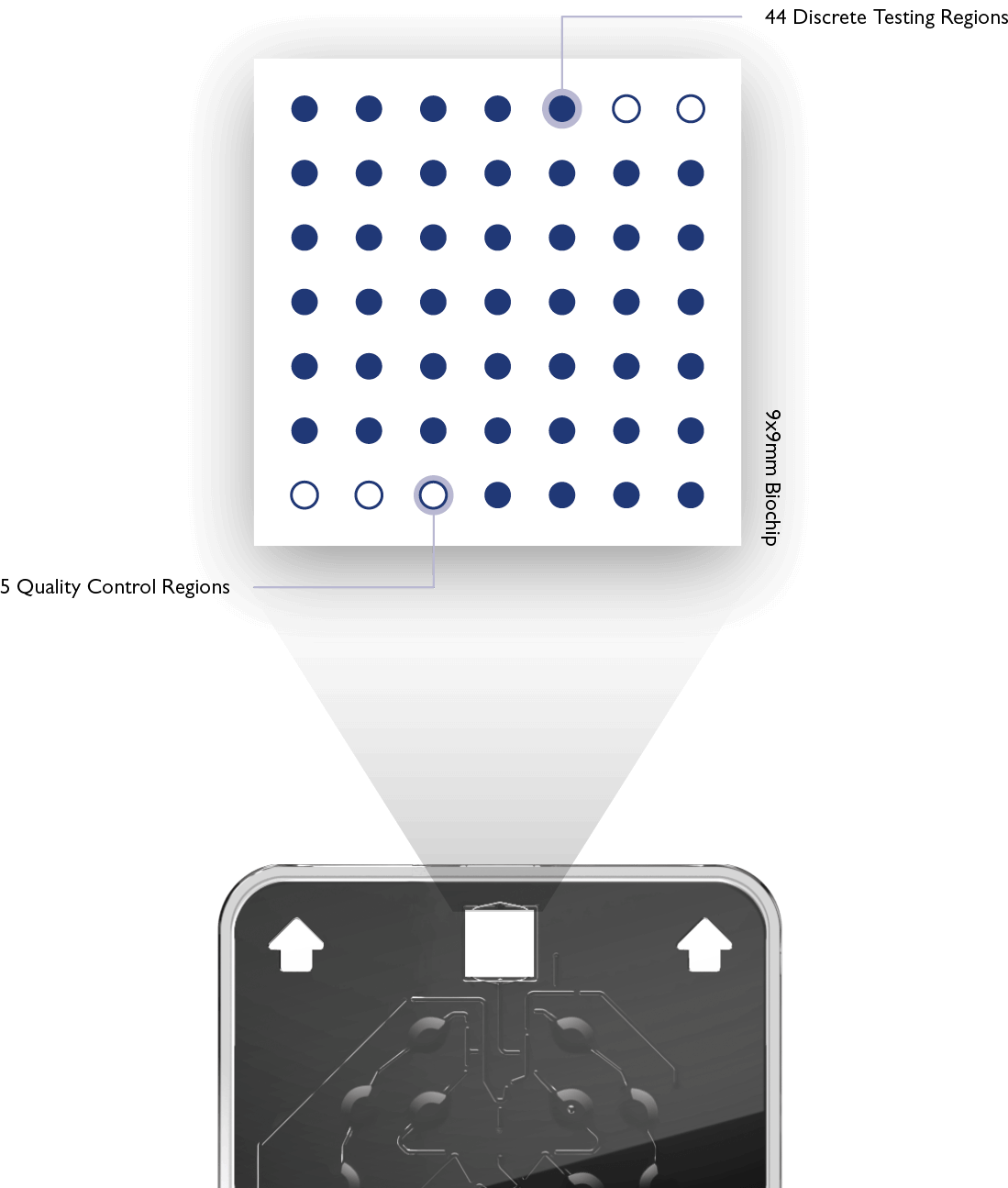
Vivalytic cartridges use microfluidics for accurate molecular diagnostics. High-Plex tests employ Randox Biochip Technology for multiple simultaneous results via end-point PCR, while Low-Plex tests use real-time PCR and melting curve analysis.
The biochip's chemiluminescent detection targets specific pathogens across discrete regions (DTRs), reducing the need for multiple assays.
Respiratory Panels
Get in touch to discover more
To find out more about our range of clinical chemistry reagents, enquire now.








