Solutions
keyboard_arrow_downServices
keyboard_arrow_downSupport & Resources
keyboard_arrow_downCompany
keyboard_arrow_downContact
Randox Health search
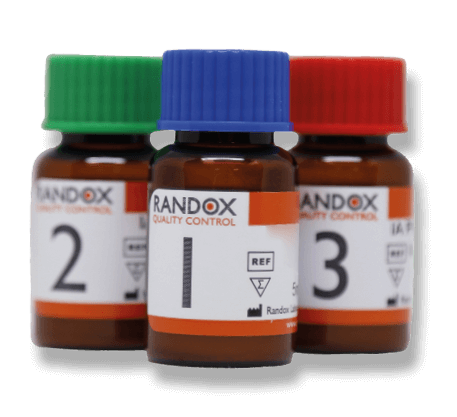
Covering 10 specialised analytes, the Acusera Immunoassay Speciality I control is designed to complement our standard immunoassay control, meeting the demands of today’s modern laboratory. Assayed target values are supplied for all 10 analytes in this true third-party control.
Get in touch to discover more
To find out more about this Acusera Control or to get in touch with your local Randox Representative, enquire now.
Features & Benefits
- Lyophilised for enhanced stability.
- 100% human serum.
- Assayed target values and ranges for a wide range of immunoassay systems.
- Stable to expiry date at 2°C to 8°C.
- Reconstituted stability of up to 5 days at 2°C to 8°C and 4 weeks at -20°C for most analytes.
| Description | Size | Cat No | |
|---|---|---|---|
| Immunoassay Speciality I Level 1 | 5 x 2ml | IAS3113 | EnquireSearch Kit InsertsView MSDSBuy Online |
| Immunoassay Speciality I Level 2 | 5 x 2ml | IAS3114 | EnquireSearch Kit InsertsView MSDSBuy Online |
| Immunoassay Speciality I Level 3 | 5 x 2ml | IAS3115 | EnquireSearch Kit InsertsView MSDSBuy Online |
Analytes
- 1-25-(OH)2-Vitamin D
- 25-OH-Vitamin D
- C-Peptide
- Insulin
- Insulin Like Growth Factor I
- Osteocalcin
- Procalcitonin
- Parathyroid Hormone (PTH)
- Anti-Thyroglobulin (Anti-TG)
- Anti-Thyroperoxidase (Anti-TPO)