Solutions
keyboard_arrow_downServices
keyboard_arrow_downSupport & Resources
keyboard_arrow_downCompany
keyboard_arrow_downContact
Randox Health search
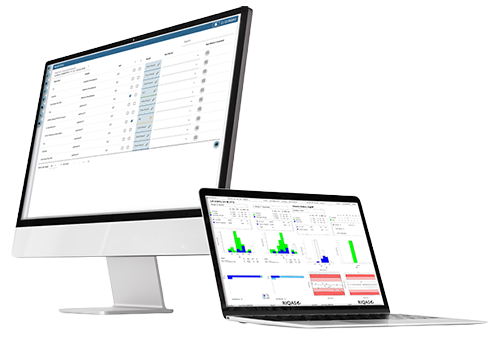
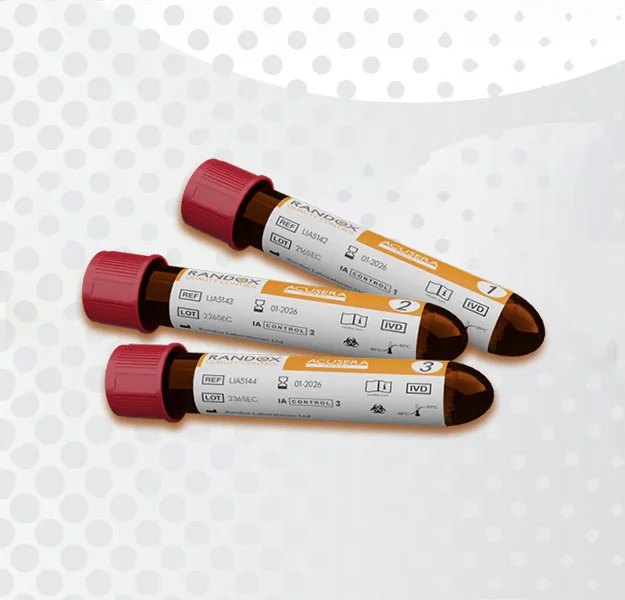
The RIQAS Serology Syphilis EQA Programme is designed to monitor the performance of Syphilis tests using immunoassay RPR, VDRL and TPHA based methods. All samples are conveniently supplied liquid ready-to-use and are suitable for both quantitative and qualitative methods of analysis
Liquid ready-to-use samples requiring minimal preparation
Monthly reporting to reduce the risk of errors
World's largest EQA scheme ensuring peer groups are maximised
Suitable for both quantitative and qualitative methods of analysis
Cycle Starts - July 2026
Not Accredited
Get in touch to discover more
To find out more about RIQAS EQA or to get in touch with your local Randox Representative, enquire now.
| Description | Frequency | Size | Cat No | |
|---|---|---|---|---|
| Serology Syphilis | Monthly | 6 x 1 ml | RQ9154 |
Parameter
- Syphilis (Methods available include immunoassay RPR, VDRL and TPHA)
Please note, product availability may vary country to country.
Related RIQAS EQA Programmes
Serology (HIV Hepatitis)
Serology (ToRCH)
Serology Epstein Barr Virus (EBV)