Solutions
keyboard_arrow_downServices
keyboard_arrow_downSupport & Resources
keyboard_arrow_downCompany
keyboard_arrow_downContact
Why use Biochip?
Prostate Cancer (PCa) is the 2nd most commonly occurring cancer in men and the 4th most common cancer overall. About in 8 men will get Prostate Cancer in their lifetime.
Histological assessment of prostate tissue obtained by Transperineal ultrasound-guided biopsy of the prostate (TURP) is commonly used in PCa diagnosis. This method has however been associated with a 75% negative biopsy rate and the procedure also associated with increased rate of UTI (15.5%). Another commonly used method of diagnosis is through histological assessment of prostate tissue obtained by Trans Rectal ultrasound-guided biopsy of the prostate (TRUS). This method however has an estimated false negative rate of 1-in-3 with three quarters reporting at least one minor complication after biopsy.
Use of the Randox PCs Biochip in primary care would:
counter_1Stratify patients into 'low' and 'high' risk categories for potential risk of PCa
counter_2Reduce the number of patients that are referred to secondary care for further investigations
counter_3Reduce the current healthcare urology burden
Randox have demonstrated novel serum combination of 4 biomarkers significantly improved the predictive potential of tPSA alone to identify patients with PCa.
| Biochip Markers | |||
|---|---|---|---|
| EGF | IL-8 | MCP-1 | iPSA |
Reduce or defer secondary care investigation referrals
to lessen the burden on healthcare waitlists for treatment

Biochip Array Technology
Utilising algorithmic probability and risk stratification to triage patients into 'low' and 'high' risk categories for potential risk of PCa
Facilitate clinical decision making
with stratification testing for patients with favourable risk disease
The Evidence MultiSTAT

Evidence MultiSTAT
The Evidence MultiSTAT is an easy to use, small footprint analyser facilitating on-site simultaneous detection of multiple biomarkers.
Using chemiluminescence as a measurement principle, the Evidence MultiSTAT consistently delivers accurate results.
With minimal sample preparation required, this versatile benchtop analyser can achieve accurate, quantitative results in minutes.
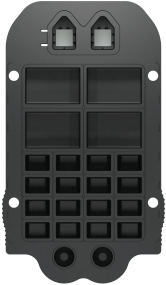
MultiSTAT Cartridge
The Evidence MultiSTAT cartridge contains the reagents required for the chemiluminescent reaction to take place incorporated into its wells.
The process from sample entry to results can be completed in 2 simple steps, with minimal risk of human error.
No other components are required.
Get in touch to discover more
To find out more about the Evidence MultiSTAT or to discuss arranging a demo, enquire now.


