RIQAS Performance Assessment – Z Score vs SDI
RIQAS Performance Assessment – Z Score vs SDI
Z Score vs SDI
You work hard to implement top class quality control in all areas of your laboratory. The success of your labours is reported to you through your External Quality Assessment (EQA) results. It can be frustrating when your report is returned, only for you to find that you’ve been assigned a poor performance score due to other laboratories in your participation group.
At RIQAS, we want your EQA results to reflect your performance, not that of everyone else, to truly illustrate the efficacy of your quality control procedures. This is why, instead of Z scores, we report your performance in terms of a Standard Deviation Index (SDI). However, we know that in some countries, you’re required to report a Z score. Don’t fret. You can still find this result in the .csv file provided with your report.
A Z score is a statistical measurement that describes a value’s relationship to the mean of a group of values. In other words, it’s a value calculated to tell us how many standard deviations (SDs) a result is from the expected mean. Z score is reported in terms of SD’s, therefore a Z score of 0 shows the result is identical to the mean.
While useful in many cases, when used in EQA, a Z score can give a false perception of performance. We want RIQAS participant performance assessment to be based on their individual performance, rather than being impacted by how well, or poorly, the other laboratories in the group performed for a sample.
Z score is calculated using a variable SD. This means that as results are added, the mean and SD can change. For example, if overall performance for a sample improves, the CV associated with the data will decrease, causing an increase in Z score. Let’s take a quick look at how RIQAS performance assessment works, and then we can get into SDI.
RIQAS Performance Assessment.
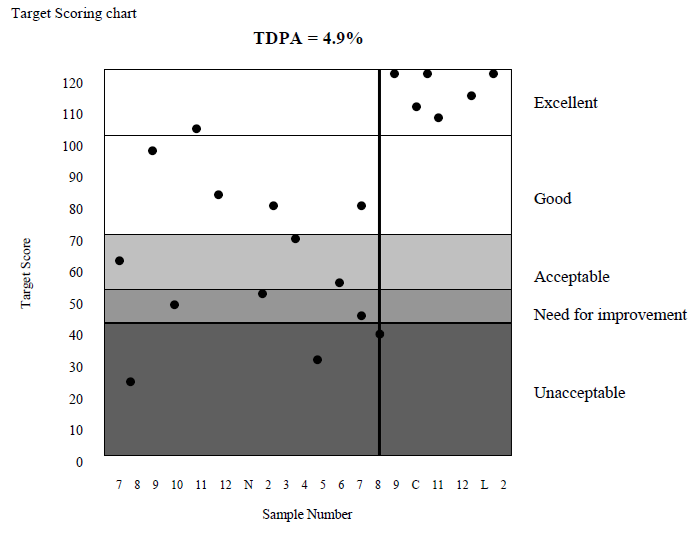
Our target scoring system has been developed to provide a simple interpretation of your laboratory’s performance. To calculate a target score, your result is calculated as a percentage deviation (V) from the Mean for Comparison. This deviation is then compared to a Target Deviation for Performance Assessment (TDPA) to calculate the Target Score.
The difference between your result and the mean for comparison is expressed as a Target Score (TS) using the following mathematical formulae:
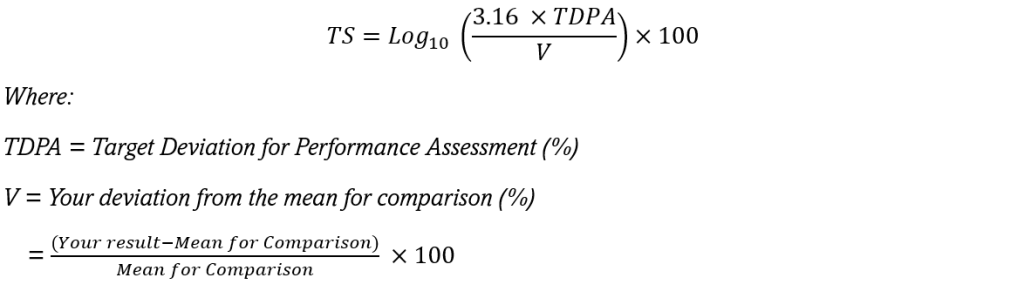
The better your percentage deviation compared to the TDPA, the higher your Target Score will be.
TDPA are set to encourage participants to achieve and maintain acceptable performance. Target Deviations are assigned to be fit-for-purpose and take all possible sources of variation into account, including sample homogeneity and stability as per ISO/IEC17043, ISO13528 and IUPAC.
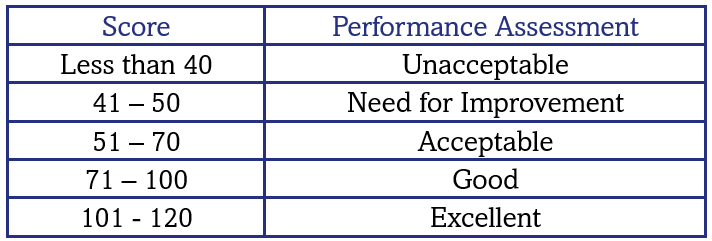
In general, the TDPA is set so that ~10% laboratories achieve Target Scores less than 50. However, depending on homogeneity and stability, the TDPAs may be adjusted, so that participants’ performance is not adversely affected by sample variability. If your % deviation (V) is equal to the Target Deviation for Performance Assessment (TDPA) then a target score of 50 is achieved.
RIQAS reviews TDPAs annually and the methods used to assign them have been agreed by the RIQAS Advisory Panel.
Standard Deviation Index (SDI)
To provide a more accurate assessment of performance, we use SDI instead of Z score. SDI is a score which compares the participant’s difference from the assigned value (mean for comparison) with an evaluation interval called the Standard Deviation for Performance Assessment (SDPA).
The SDPA calculation involves a series of steps. First, we calculate a CV for Performance assessment (CVPA) as shown below:

As mentioned, the TPDA is normally set so that ~10% of laboratories achieve a TS less than 50. In such cases, the t-value used to convert TDPA to CVPA is ~1.645. However, depending on homogeneity and stability, the TDPA may need be increased, so that participants’ performance is not adversely affected by sample variability. In such cases less than 10% of laboratories will have poor performance, and a larger t-value will be chosen to convert TDPA to CVPA
We then convert CVPA to SDPA:
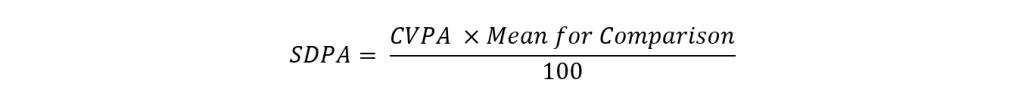
Using this equation, an initial SDPA is calculated for every mean for comparison (i.e. for all methods, method, and instrument statistics). However, for new parameters or those which have small participation numbers, it’s not always possible to assign a target deviation, TDPA or SDPA. In such cases, the SDPA will be the SD calculated when the mean for comparisons is generated.
According to ISO/IEC17043, when the assigned value is based on consensus (mean for comparison), the uncertainty of the assigned value must be calculated and combined with the SDPA when it is considered to be significant. This forms an adjusted SDPA, which is used to calculate the participant’s performance in terms of SDI.
Using the SDPAadjusted we can calculate SDI using the formula below:
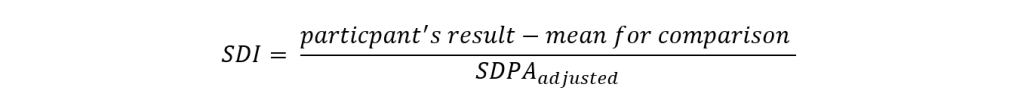
On your RIQAS report, you’ll find the SDI associated with the current sample in the text section of each report page. We also provide your last 20 SDIs, plotted on a Levey-Jennings chart, along with an indication of the mean for comparison for each sample (I = Instrument group, M = Method group, or A = All Methods group). Acceptable performance is an SDI of less than ± 2.

RIQAS EQA
RIQAS is the world’s largest EQA scheme with more than 75,000 laboratory participants spanning over 138 countries. Choosing an EQA provider is no easy task. That’s why we’ve produce a guide to help you find the right one for you. You can download it here.
At RIQAS, we’re always coming up with new ways to make your performance assessment and result interpretation even easier. We’re also proud of our new programmes and pilot schemes. This year, we’re running pilot programmes for Anti-psychotic drugs, Chagas and Blood Typing.
If you’d like to find out more about the range of programmes we provide, visit our website or download our brochure. Alternatively, you can get in touch with us at marketing@randox.com.
A Peculiar Problem in Pregnancy and the Placenta
Complications and Diagnosis of Pre-eclampsia
When we consider our most important organ its intuitive to choose the heart, the lungs or even the kidneys. However, there’s another without which none of us would be here to have the discussion. This ephemeral organ provides us with the nutrients necessary for development, removes malevolent agents, provides our initial immunity and much more, before being cast off as we enter the world. We are, of course, talking about the placenta. Indeed, all our organs work together to support life and it’s arbitrary to imbue one with more importance than the others. Nevertheless, as our first organ, the significance of the placenta is irrefutable.
Placental dysfunction, along with several other factors, is known to contribute to the development of pre-eclampsia – a complex, multisystem hypertensive disorder of pregnancy. While the aetiology of pre-eclampsia remains largely unknown, the grave complications associated with it have driven development of novel methods for predicting its onset.
Pre-eclampsia and Epidemiology
Pre-eclampsia is traditionally defined as new onset hypertension and proteinuria in pregnancy1, however, the International Federation of Gynaecology and Obstetrics’ (FIGO) clinical definition describes it as sudden onset hypertension (>20 weeks of gestation) and at least one of the following: proteinuria, maternal organ dysfunction or uteroplacental dysfunction2. It is responsible for an estimated 70’000 maternal deaths, and 500’000 foetal deaths globally3. Pre-eclampsia affects around 4% of pregnancies in the US and is more common in low-to-middle income countries (LMICs), displaying an overall pooled incidence of 13% in a cohort from sub-Saharan Africa4. The risk factors for pre-eclampsia are shown in the graphic below.

Pre-eclampsia is associated with increased morbidity and mortality worldwide. In the US, pre-eclampsia is the foremost cause of maternal death, severe maternal morbidity, maternal intensive care admissions and prematurity5.
Classical classification of pre-eclampsia included early-onset (<34 weeks gestation) and late-onset (>34 weeks gestation). However, this classification lacks clinical utility as it does not accurately illustrate maternal or foetal prognosis. Therefore, the International Society for the study of Hypertension in Pregnancy (ISSHP) and contemporary studies prefer to classify pre-eclampsia as preterm (delivery <37 weeks of gestation), term (delivery ≥37 weeks of gestation) and postpartum pre-eclampsia (after delivery).

Complications
Pre-eclampsia has been associated with acute and chronic complications for both mother and child. Worldwide risk of maternal and foetal morbidity displays adjusted odds ratios of 3.73 and 3.12, respectively (pre-eclampsia vs non pre-eclampsia)6.
Acute Maternal Complications
A range of neurological complications are associated with pre-eclampsia. The most obvious is eclampsia, defined as seizures in pregnant women commonly from 20 weeks of gestation or after birth7. Eclampsia has two proposed mechanisms: abnormal placentation reduces blood supply and causes oxidative stress, leading to endothelial damage; and elevated blood pressure in pre-eclampsia disrupts cerebral vasculature, causing hypoperfusion and damage8. In high-income countries (HICs), most women make a full recovery, however, more severe cases of eclampsia can result in permanent disability or brain damage7.
Stroke is a significant complication of pre-eclampsia, constituting 36% of strokes related to pregnancy9. The hypertension characteristic of pre-eclampsia can weaken the walls of blood vessels causing subarachnoid or intracerebral haemorrhage resulting in haemorrhagic stroke. Ischaemic stroke is also of concern due to blood clotting complications which will be discussed later.
Additonal neurological complications include visual scotoma, cortical blindness, cerebral venous sinus thrombosis, cerebral vasoconstriction syndrome and posterior reversible encephalopathic syndrome (PRES). Notably, the last three in this list frequently manifest postpartum without warning6.
HELLP (Haemolysis, Elevated Liver enzymes and Low Platelets) syndrome is a liver and blood clotting disorder and life-threatening complication of pre-eclampsia. HELLP syndrome most commonly presents immediately postpartum but can manifest any time after 20 weeks of gestation7. Microangiopathy, or small blood vessel disorder, leads to ischaemia and a subsequent increase in oxidative stress and inflammation, causing an increase in liver enzymes and participates in the initiation of HELLP. Thrombocytopenia, or platelet deficiency, is considered a product of platelet depletion resulting from heightened platelet activation triggered by widespread endothelial damage6.
Another blood clotting condition associated with pre-eclampsia is Disseminated intravascular coagulation (DIC)7, described as the dysfunction of the maternal blood clotting system resulting in multiple organ dysfunction syndrome10. DIC can cause excessive bleeding due to lack of clotting proteins, or the formation of clots due to overactive clotting proteins, ultimately causing organ damage10.
As described earlier, proteinuria is included in the diagnostic criteria for pre-eclampsia, suggesting involvement of the kidneys. This is caused by high concentrations of soluble FMS like Tyrosine kinase 1 (sFLT-1), a placental angiogenic factor, which inhibits proteins of the podocyte slit diaphragm6; the machinery involved in preventing the leakage of proteins into the urine11. Reduced levels of Vascular Endothelial Growth Factor (VEGF) and Placental Growth Factor (PlGF) stimulates Endothelin 1 expression6, known to promote podocyte detachment, further contributing to proteinuria12.
Finally, Pulmonary oedema, excessive fluid accumulation in the lungs, is an acute and life-threatening complication associated with pre-eclampsia, the likelihood of which is increased via administration of antihypertensive medications6.
Acute Neonatal Complications
There are several documented complications affecting the baby of a pre-eclamptic mother. Firstly, Intrauterine growth restriction (IUGR) can result in underdevelopment of the foetus because of deficient transfer of oxygen and other nutrients from mother to child13. This can result in low birth weight, particularly when pre-eclampsia occurs prior to 37 weeks of gestation7. In pre-eclampsia with severe symptoms, delivery frequently occurs prematurely, either spontaneously or through induction. Preterm delivery can result in complications such as neonatal respiratory distress syndrome and neonates often require ICU admission7. Additionally, there is increased risk of stillbirth in pre-eclamptic pregnancies with relative risk shown to be 1.45 (95% Cl 1.20-1.76)14. Other complications documented in neonates born through pre-eclamptic pregnancies include neonatal thrombocytopenia, bronchopulmonary dysplasia, and a range of neurodevelopment outcomes15.
Long-term Complications
The only known cure for pre-eclampsia is delivery. However, the complications for both mother and child can last long after even an uncomplicated delivery. After a pre-eclamptic pregnancy, women are increased risk of end stage renal disease (4.7-fold), stroke (4-fold) and vascular dementia (3-fold) later in life5. Women are also at increased risk of other cardiovascular disease (CVD) including chronic hypertension, coronary artery disease, congestive heart failure5, and ischaemic heart disease13. In offspring, IUGR increases the risk of development of hypertension and other CVD13. Finally, offspring have been shown to be at higher risk of increased body mass index, changes in neuroanatomy, reductions in cognitive function, and hormonal abnormalities13.

sFLT-1/PlGF ratio
The pathophysiology of pre-eclampsia is complex and enigmatic. However, placental dysfunction is known to be a factor in pre-eclampsia development. The placental-related angiogenic factors, sFLT-1 (anti-angiogenic) and PlGF (pro-angiogenic), have been implicated in this development. This ratio provides a useful measure of placental dysfunction as a sharp increase in sFLT-1 and decrease in PlGF has been shown approximately 5 weeks before onset of pre-eclampsia16.
Until recently, diagnosis of pre-eclampsia was one of clinical manifestation. However, studies such as PROGNOSIS17 and PROGNOSIS Asia18, along with others19,20, have shown strong utility of this ratio. The PROGNOSIS study showed that a ratio cutoff of ≥38 was useful for ruling out pre-eclampsia within 1 week with a negative predictive value (NPV) of 99.3% or 4 weeks with a positive predictive value (PPV) of 36.7%17. The definitions of pre-eclampsia used by ICCHP and American College of Obstetricians and Gynaecologists (ACOG) have a PPV of around 20%, but when used in combination with the sFLT-1/PlGF ratio, the PPV is enhanced to 65.5% for ruling in pre-eclampsia within 4 weeks.21.
Similar results have been shown in an Asian cohort in the PROGNOSIS Asia Study. Using the same cutoff value, this study reported an NPV of 98.9%18. Furthermore, in a sub analysis of this cohort that looked at Japanese participants, a cutoff of ≥38 displayed an NPV of 100% for ruling out pre-eclampsia within 1 week and a PPV of 32.4% for ruling in within 4 weeks22.
Accurate Identification is Essential
Like all clinical assays, those used to determine the sFLT-1/PlGF ratio are subject to rigorous quality control, essential to ensure accurate results and diagnosis. The complications of pre-eclampsia are severe and often life-threating for both mother and child. Early and accurate identification is imperative for optimal monitoring, management, and timely interventions to reduce the risk of the grave consequences associated with pre-eclampsia.
The utility of the sFLT-1/PlGF ratio has been shown over various large cohorts and provides improved identification when used in combination with established clinical definitions. While the enigma of pre-eclampsia persists, the dedication of the scientific community to unravel its complexities ensures a future where expectant mothers may benefit from more effective and tailored strategies to mitigate the risks associated with this puzzling condition. Continued research endeavours will undoubtedly shape the landscape of maternal-foetal medicine, fostering advancements that hold the promise of improved outcomes for both mothers and their unborn children.
At Randox Quality Control, we’ve introduced our Pre-eclampsia Control to the Acusera IQC range for use with in vitro diagnostic assays for the quantitative determination of PlGF and sFlt-1 in human serum and plasma.
Our true third-party Pre-eclampsia control comes with clinically relevant, assayed target values, is liquid-frozen for user convenience, utilises a human-based, commutable matrix, and has a 30-day open vial stability.
For more information on this, or any of our other controls, browse our brochure, or reach out to us today at marketing@randox.com for more information.
References
- American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in Pregnancy. Obstetrics & Gynecology. 2013;122(5):1122-1131. doi:10.1097/01.AOG.0000437382.03963.88
- Poon LC, Shennan A, Hyett JA, et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre‐eclampsia: A pragmatic guide for first‐trimester screening and prevention. International Journal of Gynecology & Obstetrics. 2019;145(S1):1-33. doi:10.1002/ijgo.12802
- Karrar SA, Hong PL. Preeclampsia. StatPearls Publishing; 2023.
- Jikamo B, Adefris M, Azale T, Alemu K. Incidence, trends and risk factors of preeclampsia in sub-Saharan Africa: a systematic review and meta-analysis. PAMJ – One Health. 2023;11. doi:10.11604/pamj-oh.2023.11.1.39297
- Rana S, Lemoine E, Granger JP, Karumanchi SA. Preeclampsia. Circ Res. 2019;124(7):1094-1112. doi:10.1161/CIRCRESAHA.118.313276
- Dimitriadis E, Rolnik DL, Zhou W, et al. Pre-eclampsia. Nat Rev Dis Primers. 2023;9(1):8. doi:10.1038/s41572-023-00417-6
- NHS. Pre-eclampsia. Health A to Z. Published September 28, 2021. Accessed January 3, 2024. https://www.nhs.uk/conditions/pre-eclampsia/complications/
- Magley M, Hinson MR. Eclampsia. StatPearls Publishing; 2023.
- Crovetto F, Somigliana E, Peguero A, Figueras F. Stroke during pregnancy and pre-eclampsia. Curr Opin Obstet Gynecol. 2013;25(6):425-432. doi:10.1097/GCO.0000000000000024
- Costello RA, Nehring SM. Disseminated Intravascular Coagulation. StatPearls Publishing; 2023.
- Kawachi H, Fukusumi Y. New insight into podocyte slit diaphragm, a therapeutic target of proteinuria. Clin Exp Nephrol. 2020;24(3):193-204. doi:10.1007/s10157-020-01854-3
- Trimarchi H. Mechanisms of Podocyte Detachment, Podocyturia, and Risk of Progression of Glomerulopathies. Kidney Dis (Basel). 2020;6(5):324-329. doi:10.1159/000507997
- Turbeville HR, Sasser JM. Preeclampsia beyond pregnancy: long-term consequences for mother and child. American Journal of Physiology-Renal Physiology. 2020;318(6):F1315-F1326. doi:10.1152/ajprenal.00071.2020
- Harmon QE, Huang L, Umbach DM, et al. Risk of Fetal Death With Preeclampsia. Obstetrics & Gynecology. 2015;125(3):628-635. doi:10.1097/AOG.0000000000000696
- Backes CH, Markham K, Moorehead P, Cordero L, Nankervis CA, Giannone PJ. Maternal Preeclampsia and Neonatal Outcomes. J Pregnancy. 2011;2011:1-7. doi:10.1155/2011/214365
- Verlohren S, Galindo A, Schlembach D, et al. An automated method for the determination of the sFlt-1/PIGF ratio in the assessment of preeclampsia. Am J Obstet Gynecol. 2010;202(2):161.e1-161.e11. doi:10.1016/j.ajog.2009.09.016
- Zeisler H, Llurba E, Chantraine F, et al. Predictive Value of the sFlt-1:PlGF Ratio in Women with Suspected Preeclampsia. New England Journal of Medicine. 2016;374(1):13-22. doi:10.1056/NEJMoa1414838
- Bian X, Biswas A, Huang X, et al. Short-Term Prediction of Adverse Outcomes Using the sFlt-1 (Soluble fms-Like Tyrosine Kinase 1)/PlGF (Placental Growth Factor) Ratio in Asian Women With Suspected Preeclampsia. Hypertension. 2019;74(1):164-172. doi:10.1161/HYPERTENSIONAHA.119.12760
- Hughes RCE, Phillips I, Florkowski CM, Gullam J. The predictive value of the sFlt‐1/PlGF ratio in suspected preeclampsia in a New Zealand population: A prospective cohort study. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2023;63(1):34-41. doi:10.1111/ajo.13549
- Nikuei P, Rajaei M, Roozbeh N, et al. Diagnostic accuracy of sFlt1/PlGF ratio as a marker for preeclampsia. BMC Pregnancy Childbirth. 2020;20(1):80. doi:10.1186/s12884-020-2744-2
- Verlohren S, Brennecke SP, Galindo A, et al. Clinical interpretation and implementation of the sFlt-1/PlGF ratio in the prediction, diagnosis and management of preeclampsia. Pregnancy Hypertens. 2022;27:42-50. doi:10.1016/j.preghy.2021.12.003
- Ohkuchi A, Saito S, Yamamoto T, et al. Short-term prediction of preeclampsia using the sFlt-1/PlGF ratio: a subanalysis of pregnant Japanese women from the PROGNOSIS Asia study. Hypertension Research. 2021;44(7):813-821. doi:10.1038/s41440-021-00629-x
UKAS ISO15189:2022 Transition Update
Throughout 2023, UKAS have been hard at work training Assessment Managers and Technical Assessors on the new requirements of the updated ISO15189 guidelines, sharing information about the updated standard and developing the UKAS 15189:2022 Transition Hub providing a one-stop-shop for information on the ISO15189:2022 update.
Recently, UKAS have published a Transition update to remind laboratories of where they stand in seeking their updated accreditation. In this update, UKAS state “As per the UKAS transition plan, all assessments due to take place from the 1st January 2024 will be to ISO15189:2022.”
A gap analysis will be required one month prior to transition assessments, detailing the gaps and the actions which have been taken to remedy these gaps. This should include evidence, such as updated documents and records, embedded in the gap analysis document, showing what action has been taken to bring a laboratory’s practices in line with the updated standard.
An important note included in this transition update is , “UKAS cannot grant accreditation on intent; organisations shall make the necessary changes and have implemented these prior to the transition assessment.” So if your accreditation assessment is due soon, you might want to make use of our ISO15189:2022 Accreditation Guide to assist you in your gap analysis to ensure you don’t miss out.
This is crucial for laboratories because failure to align with the 2022 version of the standard before the deadline of 6th December 2025 will result in a suspension of ISO15189 accreditation for up to 6 months.
Some of the key accreditation updates include:




Randox Quality Control’s Acusera range provides true third part quality controls designed to help you achieve all aspects of ISO15189:2022 accreditation including commutable matrices containing consistent, clinically relevant concentrations with unrivalled consolidation of analytes. To learn more about our range of quality control products, visit our website or, get in touch today at marketing@randox.com
Introducing Comprehensive Educational Guides on Updated CLIA Proficiency Testing Regulations
We are thrilled to present two educational guides that delve into the newly updated minimum performance specifications for Proficiency Testing by CLIA (Clinical Laboratory Improvement Amendments). These regulations, set to be implemented by 2024, aim to enhance the accuracy and reliability of test results in clinical laboratories. Here, we introduce these invaluable resources designed to assist laboratories in navigating the evolving landscape of proficiency testing.
1. Proficiency Testing Regulations Related to Analytes and Acceptable Performance – A Final Rule (Microbiology):
Our first guide focuses on the specific regulations and requirements pertaining to microbiology proficiency testing. With a comprehensive exploration of these guidelines, this guide is a useful resource for microbiology labs striving to ensure precision and integrity in their testing procedures. From the required categories of testing to maintaining optimal testing conditions, the guide details the updates that promote adherence to the highest standards of quality and safety.
2. Proficiency Testing Regulations Related to Analytes and Acceptable Performance – A Final Rule (Non-Microbiology):
For non-microbiology laboratories, our second guide delves into the updated proficiency testing regulations concerning various analytes. From chemistry to haematology, molecular diagnostics to immunology, this guide offers a comprehensive overview of the new requirements and minimum performance specifications. By embracing these regulations, medical laboratories can uphold the utmost accuracy and reliability in their test results, ensuring optimal patient care and clinical decision-making.
Elevating Laboratory Practices:
These educational guides are indispensable tools that empower laboratories to navigate the changing landscape of proficiency testing regulations. By staying informed and adopting the updated minimum performance specifications, laboratories can maintain compliance, demonstrate excellence, and ultimately deliver the highest quality of care to their patients.
Accessing the Guides:
We invite you to access these comprehensive educational guides by following the link provided below. They offer a wealth of knowledge and practical insights, serving as essential references for laboratory professionals, quality managers, and anyone involved in clinical diagnostics.
With the implementation of updated CLIA proficiency testing regulations on the horizon, these educational guides come at a crucial time. By embracing the knowledge and guidance they provide, laboratories can navigate the changing landscape with confidence and ensure their adherence to the highest standards of proficiency testing. Together, let’s strive for excellence, precision, and patient-centric care in clinical laboratory practices.
#CLIARegulations #ProficiencyTesting #ClinicalLaboratories #QualityAssurance #PatientCare

QC Industry Articles & White Papers
A simple swab test developed by Randox scientists could help curb the antibiotic resistance pandemic.
The test, which can rapidly detect and identify the cause of 21 respiratory infections in just 5 hours, can subsequently determine the appropriate antibiotic drug treatment for patients.
Following the Lancet Infectious Diseases report last week that antibiotic resistance is growing at a rapid rate, with many ‘superbugs’ completely unresponsive to antibiotics, it is more important than ever that antibiotics are only prescribed when they will effectively treat an infection.
With 70% of GPs admitting that they prescribe antibiotics when they are unsure if they are treating a viral or bacterial infection, many patients are therefore taking antibiotics when they are ineffective in treating viral infections.
Given that only bacterial infections can be treated with antibiotics, rapid diagnostic tests are urgently needed to identify bacterial and viral infections.
Professor Peter Coyle, who leads one of the UK’s Regional Virology Laboratories in Belfast diagnosing infectious diseases, warns against antibiotic misuse:
“The commonest types of respiratory infection are of viral origin and antibiotics are ineffective in their treatment. Improving the diagnosis of respiratory infections is an important goal in improving patient management and reducing antimicrobial resistance. The threat of antimicrobial resistance and the loss of effective antibiotics has become a major and growing concern in health care provision.”
The new rapid and accurate test will give both patient and GP confidence of their diagnosis of respiratory infections and will allow for quicker treatment if necessary.
Making this test available through GPs would have additional efficiency savings for the NHS, by eliminating the need for lengthy microbiology lab tests and unnecessarily prescribing drugs which are not needed.
John Lamont, Chief Scientist at Randox Laboratories, whose team developed the test, commented;
“Current diagnostic testing for respiratory infections take at least 36 hours to confirm the nature of an infection, and they cannot name and categorise infections as bacterial or viral in the way that this new respiratory test can. C-reactive protein tests, for example, that are currently in use can only indicate whether a bacterial infection is likely. We need more than just guess work to combat the antibiotic resistance pandemic.”