Alzheimer’s Disease, ApoE & Risk Detection
Alzheimer’s Disease, ApoE & Risk Detection
Alzheimer’s Disease, ApoE4 & Risk Detection
Alzheimer’s disease touches all of us, whether directly through an affected loved one or through its frequent presence in the news. Alzheimer’s disease is a progressive neurodegenerative disorder characterised by cognitive decline, memory loss, and functional impairments. It is the most common cause of dementia, affecting millions of individuals worldwide 1 and posing significant challenges to healthcare systems. As the global population ages, the prevalence of Alzheimer’s disease is expected to rise, highlighting the urgent need for effective diagnostic and therapeutic strategies.
The current methods used to diagnose Alzheimer’s disease consist of clinical assessment and supporting neuroimaging techniques which can be expensive and, in some cases, fail to provide a definitive diagnosis at the early stages required to facilitate timely intervention and slow disease progression.
With novel therapeutics which aim to slow the progression of Alzheimer’s Disease achieving approval in the United States and being considered for approval in other countries, diagnostics which can identify those at risk of developing this disease are more important than ever. Biomarkers have emerged as vital tools in the early detection and risk assessment of Alzheimer’s disease. Among these, the apolipoprotein E gene (ApoE) has garnered significant attention. The apolipoprotein E protein (ApoE) exists in three common isoforms: ApoE2, ApoE3, and ApoE4. These isoforms combine to form six common genotypes in the general population. Notably, the presence of the ApoE4 allele is associated with a significantly increased risk of developing Alzheimer’s disease.
The Randox ApoE4 Array marks a significant advancement in Alzheimer’s disease biomarkers. This quick and sensitive blood test allows direct ApoE4 genotyping, eliminating the need for traditional molecular techniques. With its fast and accurate results, healthcare providers can efficiently assess an individual’s genetic risk for Alzheimer’s disease.
In this article, we present a summary of our latest whitepaper: Alzheimer’s Disease, ApoE4 & Risk Detection in which we explore the critical role of ApoE genotyping in Alzheimer’s Disease, the innovative technology behind the Randox ApoE4 Array, and its implications for clinical practice. You can download this whitepaper through the link below.
Apolipoprotein E and Alzheimer’s Risk
The ApoE gene transcribes a 229 amino acid protein which primarily functions to mediate lipid transport in the brain and periphery. ApoE is also involved in immune modulation, synapse regeneration, and the clearance/degradation of amyloid-β, a peptide crucial to the development of Alzheimer’s disease2.
There are 3 common isoforms of the human ApoE, differentiated through single nucleotide polymorphisms (SNPs) at amino acid positions 112 and 1582:

These three isoforms combine to produce six common genotypes: E2/E2, E2/E3, E2/E4, E3/E3, E3/E4, and E4/E4. Each genotype is associated with a different level of risk for developing Alzheimer’s disease, with the ApoE4 and ApoE2 isoforms presenting the highest3 and lowest risk 4 respectively.
The structural differences among ApoE isoforms affect their ability to bind lipids, receptors, and amyloid-β, influencing cognitive decline. ApoE2 and ApoE3 bind effectively to HDL (High density lipoprotein), while ApoE4 binds to VLDL (Very low-density lipoprotein), resulting in poor lipidation and toxic aggregates1,2. Cholesterol is crucial for brain function, supporting membrane integrity, signal transduction, and amyloid-β regulation. The interaction of ApoE with amyloid-β regulates amyloid plaque formation, influencing Alzheimer’s disease onset. Cholesterol must be converted to 24S-hydroxycholesterol to cross the blood-brain barrier. Poor cholesterol and phospholipid transport by ApoE4 increases the risk of late-onset Alzheimer’s disease in E4 carriers2.
Randox ApoE Array
The Randox ApoE4 Array is a rapid and highly sensitive blood test that facilitates direct ApoE4 genotyping without the need for traditional molecular techniques. It measures both total ApoE and ApoE4 protein levels directly from a plasma sample, using the ApoE4/total ApoE ratio to classify the ApoE4 status as negative or positive. Additionally, the array can distinguish between heterozygous and homozygous individuals among ApoE4 positive samples, aiding in the assessment of Alzheimer’s disease risk.
Using Randox proprietary chemiluminescent biochip-sandwich immunoassays, the array provides accurate results within three hours from a minimally invasive plasma sample. This method has shown 100% concordance with genotypes achieved through restriction fragment length polymorphism (RFLP) in two separate centres5. Compared to other methods like isoelectric focusing, mass spectrometry, bead-based immunoassay, Sandwich ELISA, and PCR, the Randox ApoE Array offers advantages in speed, simplicity, and automation.

Clinical Implications
The Randox ApoE Array offers significant benefits for managing Alzheimer’s disease through early detection and personalised treatment plans. This minimally invasive blood test identifies individuals at higher genetic risk for Alzheimer’s Disease enabling:
- Timely Interventions: Early identification allows for preventive measures and lifestyle modifications, such as cognitive training and increased physical activity, to delay symptom onset.
- Regular Monitoring: High-risk individuals can be monitored for cognitive changes, enabling early intervention for mild cognitive impairment.
Personalised Treatment Plans
Accurate ApoE genotyping supports personalised treatment:
- Targeted Therapies: ApoE phenotype informs the selection of therapies, especially for ApoE4 carriers.
- Risk Stratification: Patients can be stratified by genetic risk for targeted preventive measures.
- Optimised Medication: Genotype information guides medication choices, enhancing efficacy.
- Family Counselling: Genotyping aids family counselling, advising on preventive measures and monitoring.
Conclusions
The Randox ApoE Array represents a groundbreaking advancement in the early detection and management of Alzheimer’s disease. By providing a rapid, highly sensitive, and minimally invasive method for ApoE genotyping, it empowers healthcare providers to implement timely interventions and personalised treatment plans. This innovative approach not only enhances the accuracy of Alzheimer’s risk assessment but also supports the development of targeted therapies and optimised medication regimens, ultimately improving patient outcomes.
Early detection of Alzheimer’s disease allows for proactive measures that can delay the onset of symptoms, while personalised treatment strategies tailored to an individual’s genetic profile offer a more effective management approach. Furthermore, the array’s capability to provide real-time insights into a patient’s disease stage makes it an invaluable tool in the fight against Alzheimer’s disease.
For a deeper understanding of the critical role of ApoE genotyping in Alzheimer’s disease and the innovative technology behind the Randox ApoE Array, we invite you to download our comprehensive whitepaper be clicking on the image below. You can also visit our website to access this and other valuable resources and to learn more about the Randox ApoE4 Array.
References
- Raulin AC, Doss S V., Trottier ZA, Ikezu TC, Bu G, Liu CC. ApoE in Alzheimer’s disease: pathophysiology and therapeutic strategies. Mol Neurodegener. 2022;17(1):72. doi:10.1186/s13024-022-00574-4
- Husain MA, Laurent B, Plourde M. APOE and Alzheimer’s Disease: From Lipid Transport to Physiopathology and Therapeutics. Front Neurosci. 2021;15. doi:10.3389/fnins.2021.630502
- Qian J, Wolters FJ, Beiser A, et al. APOE-related risk of mild cognitive impairment and dementia for prevention trials: An analysis of four cohorts. PLoS Med. 2017;14(3):e1002254. doi:10.1371/journal.pmed.1002254
- Reiman EM, Arboleda-Velasquez JF, Quiroz YT, et al. Exceptionally low likelihood of Alzheimer’s dementia in APOE2 homozygotes from a 5,000-person neuropathological study. Nat Commun. 2020;11(1):667. doi:10.1038/s41467-019-14279-8
- Badrnya S, Doherty T, Richardson C, et al. Development of a new biochip array for APOE4 classification from plasma samples using immunoassay-based methods. Clinical Chemistry and Laboratory Medicine (CCLM). 2018;56(5):796-802. doi:10.1515/cclm-2017-0618
Active vs Total Vitamin B12
Total vs Active B12
Vitamin B12, or cobalamin, is a vital water-soluble vitamin that plays an essential role in myelination initiation and development, cellular energy and fatty acid metabolism. It is a cofactor for enzymes methionine synthase and L-methyl-malonyl-coenzyme A mutase and, in addition to folate, is essential for DNA and protein synthesis. In the UK, up to 6% of adults under 60 have been diagnosed with Vitamin B12 deficiency and figures are much higher in elderly populations1. Additionally, these data do not consider the high rates of missed diagnosis associated with B12 deficiency, which some reports claim to be as high as 26%2. New guidance from the National Institute of Health and Care Excellence (NICE) advise that Active vitamin B12 testing is recommended for some groups of patients. In this article, we’ll look at this essential vitamin, B12 deficiency and the associated complications, compare the biomarkers used to diagnose B12 deficiency, and finally, present the new Acusera Active B12 Control.
Aetiology
Vitamin B12 deficiency can arise due to dietary insufficiency, malabsorption resulting from damage to the small intestine, often caused by conditions like Coeliac disease or Crohn’s disease, or via pernicious anaemia – an autoimmune condition which results in an inability to absorb vitamin B12.
It is a common problem in the elderly population – bodily stores of vitamin B12 can take up to 20 years to become depleted, meaning complications have often already begun before diagnosis occurs. The most common source of vitamin B12 comes from dietary intake of animal products therefore vegetarian dietary requirements are considered a considerable risk factor for vitamin B12 deficiency.
Pathophysiology and Complications
Vitamin B12 deficiency significantly impacts health, affecting various bodily functions, potentially leading to a range of complications. Megaloblastic anaemia is a common complication associated with vitamin B12 deficiency and is characterised by the presence of large red blood cell precursors (megaloblasts) in the bone marrow3. The lack of vitamin B12 results in impaired DNA synthesis and an inhibition of nuclear division. However, cytoplasmic maturation is less effected. This results in asynchronous maturation of the nucleus and cytoplasm in erythrocytes and causes the synthesis of abnormally large megaloblasts. This causes the cessation of DNA synthesis and DNA replication errors, culminating in apoptotic cell death. Common symptoms of megaloblastic anaemia include weakness, shortness of breath, palpitations, tachycardia, Hunter glossitis or splenomegaly3.
Pernicious anaemia is a condition commonly associated by vitamin B12 deficiency. Pernicious anaemia is an autoimmune disorder which affects the gastric mucosa resulting in impaired absorption of vitamin B12. Common symptoms of pernicious anaemia include glossitis, hair loss, dry skin, memory loss, poor concentration, poor sleep, confusion and dizziness, shortness of breath, Diarrhoea, indigestion, loss of appetite, mood swings and suicidal thoughts.
Neurological issues may also arise, including numbness, mobility loss, and memory issues, and in some cases, depression4. Additionally, B12 deficiency is linked to increased risks of cardiovascular events5, infertility6, and autoimmune diseases like multiple sclerosis7 and lupus8. In children, vitamin B12 deficiency can manifest as failure of brain and overall growth and development, developmental regression, hypotonia, lethargy, hyperirritability, or coma9.

Active B12 as a marker of Deficiency
There are several markers of vitamin B12 deficiency. The most used in clinical practice are total vitamin B12, homocysteine, methylmalonic acid (MMA), and Holotranscobalamin (HoloTC) – also known as Active B12. HoloTC accounts for between 10-30% of total B12 and is the metabolically active form of vitamin B12.
When compared with total B12 quantification, HoloTC measurement has been shown to be a more sensitive and specific biomarker of B12 deficiency, particularly at borderline clinical levels10, in various cohorts11,12 including those on vegan diets13 – a known risk factor for B12 deficiency. Furthermore, HoloTC was shown to provide the higher diagnostic accuracy in clinical and subclinical B12 deficiency versus Total B12, MMA and homocysteine with significantly higher accuracy in women over 5011 – a population at high risk of B12 deficiency.
In response to the mounting evidence of the superior utility of HoloTC quantification, the National Institute for Health and Care Excellence (NICE) have produced new guidelines recommending either total B12 or HoloTC for the initial testing of suspected vitamin B12 deficiency. These guidelines specify the use of active B12 during pregnancy and suggest that active B12 might provide a more specific assessment in certain clinical contexts.
Acusera Active B12 Control
For the reasons stated above, Randox are proud to present the Acusera Active Vitamin B12 Control. This control is designed for use with in vitro diagnostic assays for the quantitative determination of HoloTC in human serum and plasma and is suitable for use on a variety of analysers. This true third-party control is provided in a liquid ready-to-use format reducing preparation time and has an impressive 30-day open vial stability, helping to minimise waste. Like all Acusera controls, the Active B12 Control is supplied at consistent, clinically relevant levels to ensure the test system is challenged at the critical decision limits used to aid diagnosis. Furthermore, this control is provided with assayed target values for a range of analysers which are available through our new SmartDocs portal.
Summary of Benefits:
- Dedicated, HoloTC control.
- 30-day Open Stability.
- 2-year shelf life.
- Liquid Ready-to-use.
- Human Serum Based.
- Consistent, clinically significant values.
- True third-party controls.
- Assayed target values.
Ensure the accuracy of your vitamin B12 testing with Randox’s Acusera Active Vitamin B12 Control. Join the other laboratories around the world who trust Acusera to help deliver reliable, clinically relevant test results. Contact us today at marketing@randox.com to learn more and order your supply of the Acusera Active B12 Control.
References
- Hunt A, Harrington D, Robinson S. Vitamin B12 deficiency. BMJ. 2014;349(sep04 1):g5226-g5226. doi:10.1136/bmj.g5226
- Oh RC, Brown DL. Vitamin B 12 Deficiency Clinical Manifestations of Vitamin B 12 Deficiency. Vol 67.; 2003. www.aafp.org/afp
- Hariz A, Bhattacharya PT. Megaloblastic Anemia. StatPerals Publishing; 2024.
- Patel S V., Makwana AB, Gandhi AU, Tarani G, Patel J, Bhavsar V. Factors associated with vitamin B12 deficiency in adults attending tertiary care Hospital in Vadodara: a case control study. Egypt J Intern Med. 2022;34(1):11. doi:10.1186/s43162-022-00104-0
- Pawlak R, Parrott SJ, Raj S, Cullum-Dugan D, Lucus D. How prevalent is vitamin B12 deficiency among vegetarians? Nutr Rev. 2013;71(2):110-117. doi:10.1111/nure.12001
- Green R, Graff JP. Megaloblastic Anemia. In: Atlas of Diagnostic Hematology. Elsevier; 2021:47-51. doi:10.1016/B978-0-323-56738-1.00004-X
- Najafi MR, Shaygannajad V, Mirpourian M, Gholamrezaei A. Vitamin B(12) Deficiency and Multiple Sclerosis; Is there Any Association? Int J Prev Med. 2012;3(4):286-289.
- Segal R, Baumoehl Y, Elkayam O, et al. Anemia, serum vitamin B12, and folic acid in patients with rheumatoid arthritis, psoriatic arthritis, and systemic lupus erythematosus. Rheumatol Int. 2004;24(1):14-19. doi:10.1007/s00296-003-0323-2
- Stabler SP. Vitamin B12 Deficiency. New England Journal of Medicine. 2013;368(2):149-160. doi:10.1056/NEJMcp1113996
- Bondu JD, Nellickal AJ, Jeyaseelan L, Geethanjali FS. Assessing Diagnostic Accuracy of Serum Holotranscobalamin (Active-B12) in Comparison with Other Markers of Vitamin B12 Deficiency. Indian Journal of Clinical Biochemistry. 2020;35(3):367-372. doi:10.1007/s12291-019-00835-y
- Jarquin Campos A, Risch L, Nydegger U, et al. Diagnostic Accuracy of Holotranscobalamin, Vitamin B12, Methylmalonic Acid, and Homocysteine in Detecting B12 Deficiency in a Large, Mixed Patient Population. Dis Markers. 2020;2020:1-11. doi:10.1155/2020/7468506
- Verma A, Aggarwal S, Garg S, Kaushik S, Chowdhury D. Comparison of Serum Holotranscobalamin with Serum Vitamin B12 in Population Prone to Megaloblastic Anemia and their Correlation with Nerve Conduction Study. Indian Journal of Clinical Biochemistry. 2023;38(1):42-50. doi:10.1007/s12291-022-01027-x
- Lederer AK, Hannibal L, Hettich M, et al. Vitamin B12 Status Upon Short-Term Intervention with a Vegan Diet—A Randomized Controlled Trial in Healthy Participants. Nutrients. 2019;11(11):2815. doi:10.3390/nu11112815
Combating Gastroenteritis – Advanced Diagnostic Techniques for Effective Management

Gastroenteritis, often referred to as stomach flu or a stomach bug, affects millions globally each year with symptoms such as diarrhoea, vomiting, abdominal pain, and fever. It is primarily caused by viral and bacterial infections, with rotavirus, norovirus, and Clostridium difficile being the main culprits.
At Randox, we’re dedicated to improving healthcare worldwide. That’s why we’ve produced an educational guide on gastroenteritis and the latest advancements in diagnostic techniques, including a range of novel gastroenteritis test for the Vivalytic POCT system. In this blog, we’ll look at a few of the key points raised in our latest educational guide. You can download this educational guide by clicking the below.
Why Gastroenteritis Matters
Gastroenteritis can lead to severe dehydration, especially in vulnerable groups like children and the elderly. It spreads mainly through the faecal-oral route, which includes consuming contaminated food and water. Prompt and accurate diagnosis is crucial for effective management.
Key Pathogens
Rotavirus
Rotavirus is a major cause of severe gastroenteritis in children. Highly contagious, it leads to rapid dehydration, making rehydration and supportive care essential. Vaccines like Rotarix and RotaTeq are effective in preventing infections.
Norovirus
Norovirus is responsible for most viral gastroenteritis outbreaks. Extremely contagious, it spreads quickly through direct contact and contaminated food. Symptoms include sudden vomiting and diarrhoea, often leading to dehydration. While there’s no specific treatment, staying hydrated is key.
Clostridium difficile
Clostridium difficile, or C. diff, is a leading cause of antibiotic-associated diarrhoea, particularly in healthcare settings. It produces toxins that cause inflammation and damage to the colon, requiring targeted antibiotic treatment for severe cases.
Advanced Diagnostics: The Vivalytic System
Accurate and timely detection of gastroenteritis pathogens is crucial for effective patient management. The Vivalytic Point of Care Testing (POCT) system, developed by Bosch Healthcare Solutions and Randox Laboratories, offers rapid and reliable diagnostics. This system helps healthcare professionals make quicker decisions, improving patient outcomes.
The Vivalytic Gastroenteritis Panels
The Vivalytic panels detailed in our guide include tests for rotavirus, norovirus, and Clostridium difficile. These panels utilise advanced molecular techniques to provide quick and accurate results, helping to streamline the diagnosis process and enhance patient care. By using these panels, healthcare providers can efficiently identify the specific pathogens responsible for gastroenteritis, allowing for targeted treatment and improved patient outcomes.
Features of the Vivalytic System
The Vivalytic system is user-friendly and efficient. It supports both High-Plex and Low-Plex testing, allowing for the simultaneous detection of multiple pathogens from a single sample. This versatility makes it an invaluable tool for healthcare professionals.
Conclusion
Gastroenteritis, caused by pathogens like rotavirus, norovirus, and Clostridium difficile, presents significant health challenges. Advanced diagnostic technologies, such as the Vivalytic system, are crucial in managing and controlling this condition. For a comprehensive understanding of gastroenteritis and innovative diagnostic techniques, download our detailed educational guide.
For more information on the Vivalytic, the panels mentioned, or any of our products, don’t hesitate to reach out to us at marketing@randox.com
Serum Indices – Product Spotlight

Errors can occur at any point in the pre-analytical, analytical, or post-analytical stages of a diagnostic test. It is general practice for errors in the analytical stage to be identified through quality control procedures. However, pre-analytical errors are often treated with less importance than those in later stages of testing. Interference caused by haemolysis, icterus and lipemia (HIL) are common forms of pre-analytical error which affect assay methods, yielding erroneous results. The Randox Acusera Serum Indices (SI) control is designed to monitor an IVD instrument’s response in the detection of HIL interferences.
HIL interference is not novel and has been historically identified through a series of visual assessments. While haemolytic, icteric and lipemic interference causes a visual change in the sample, these methods are not quantitative and are subject to interpretation by laboratory professionals. Modern analysers have built-in capabilities for the automated detection of HIL interference which can quantitatively or semi-quantitatively measure haemolysis, icterus and lipemia, and provide and an index for each. This data can then be used to determine if a sample should be accepted for testing or rejected due to intrinsic interference.
The pre-analytical phase of laboratory testing includes collection, handling, transportation, storage, and preparation of samples. Even when the highest level of care is taken to ensure that all aspects of the pre-analytical phase are suitable and correct, errors can occur, exhibiting the need for clear and efficient quality control processes.
As part of our Acusera quality control range, Randox has developed the Serum Indices quality control to aid in the detection of the common pre-analytical error’s haemolysis, icterus and lipemia, collectively known as HIL. HIL interference can have disastrous effects on the quantification of many analytes, and it is therefore vital to determine levels of interference to improve laboratory efficiency and reduce the frequency of erroneous results.

The graph below shows the wavelengths at which each of these interferents may affect assays and the table below describes these forms of interference:

Classical determination of HIL interference took the form of a visual assessment. A sample was examined for tell-tale signs of one or more of these types of interference. However, these methods are subject to operator interpretation and lack harmonisation and uniformity across the industry. These signs are detailed in the table and illustrated in the graphic below:


Modern clinical chemistry analysers have onboard HIL detection capabilities which offer objective, semi-qualitative or qualitative analysis of these forms of interference in a more precise and consistent manner. Automation of HIL detection improves laboratory throughput along with test turnaround times and enhances the reportability of the results.
Errors at any stage of the analytical process will result in retesting of the sample. Errors in the pre-analytical phase can have repercussions such as increased cost of repeated sample collection and testing, poor test turnaround times, and more seriously, delayed or incorrect diagnosis causing an exacerbation in the condition of the patient. To add to the adverse outcomes on patients, repeated testing places additional stress on laboratory resources and staff which ultimately affects every aspect of a laboratory’s daily activities.
To correctly analyse HIL interference, absorbance readings at different strategically selected wavelengths supplement the calculation of the interference indices. C56-A recommends laboratories consider several parameters when selecting an HIL interference analysis method:

Before implementing results obtained from any method detecting HIL in patient samples, it is imperative to evaluate the specificity and sensitivity of the method at a minimum of two clinically relevant concentrations. This assessment should encompass the sensitivity of the icterus index to haemoglobin and lipids, the haemolysis index to bilirubin and lipids, and the lipemic index to haemoglobin and bilirubin.
In instances of HIL interference, laboratories bear the responsibility of managing the associated results and samples. It is crucial never to utilise an HIL index for the correction of patient results. Typically, if a sample is determined to be affected by one or more of these interferences, the laboratory should reject the result and appropriately dispose of the sample. Nonetheless, in certain scenarios, threshold values can be established. For instance, haemolysis may exert a lesser impact on samples with elevated analyte concentrations. In such cases, laboratories may opt for a distinct procedure in handling these results compared to those exhibiting haemolytic interference at lower analyte concentrations.
Acusera Serum Indices Control
The Randox Acusera Serum Indices (SI) control is designed to be used to monitor an IVD instrument’s response in the detection of haemolyzed, icteric and lipemic (HIL) samples. This control can be utilised in laboratory interference testing to assist in improving error detection of pre-analytical errors affecting clinical chemistry testing. This control provides a full range of clinically relevant testing levels, including a negative (-) and three positives (+, ++ & +++).
The Randox Control offers a comprehensive solution with 3 levels for each form of interference and a negative control, providing a wider coverage compared to alternatives in the market. Our product is conveniently supplied in a lyophilized format, ensuring an extended shelf-life and ease of storage. Customers appreciate the stability of our control, as it consistently meets the 14-day open stability claims, minimizing waste and optimizing laboratory efficiency.
Typical Values


RIQAS Serum Indices External Quality Assessment
The RIQAS Serum Indices EQA programme is designed for the pre-analytical assessment of Haemolytic, Icteric and Lipemic (HIL) interferences. Available in a bi-monthly format with the option to report either quantitative or semi-quantitative results for the HIL parameters, this programme also provides an assessment on how these interferences impact on up to 25 routine chemistry parameters. This provides invaluable information on whether a correct judgement is being made to report results.
• Lyophilised for enhanced stability
• Human based serum ensuring commutable sample matrix
• Bi-monthly reporting
• HIL parameters include the option of quantitative or semi-quantitative reporting
• Interpretation of chemistry parameter results
• Submit results and view reports online via RIQAS.net

How can Randox help?
It is crucial laboratories test for haemolysis, icterus and lipemia to ensure the accuracy of their test processes are maintained. ISO 15189:2022 promotes the identification and control of non-conformities in the pre-analytical process, therefore, using Randox Serum Indices control and RIQAS Serum Indices EQA will help laboratories fulfil the requirements of the new edition of this standard.
Randox Serum Indices control displays improved consolidation, stability, and commutability to ensure laboratories are equipped to accurately determine pre-analytical interferences. Our Serum Indices control can be used with most major chemistry analysers including Roche, Abbot, Beckman, Ortho, and Siemens. When used in conjunction with Acusera 24.7, this control offers laboratories the ability to compare their HIL results with their peer group and identify potential failures in their pre-analytical process.
Simply send us an email by clicking the link below and we will get in touch!
Medical Laboratory Professionals Week 2024
Medical Laboratory Professionals Week (MLPW) is recognised every year in the last full week of April. It’s an opportunity to increase the public understanding of, and appreciation for, the hard work of clinical laboratory staff around the world. It’s also an opportunity to inject a little fun into the laboratory. So, this year, we’ve created a Lab Professionals QC Bingo card. Have a go and see how many your laboratory can get!
How many boxes does your lab tick?

If you’re calling Bingo! you must be an Acusera 24.7 customer. If not, keep reading to find out how you can make daily life in your laboratory more straightforward.
What are Medical Laboratory Professionals?
Medicine wouldn’t be where it is today without the work of these laboratory professionals. They’re on the frontline. Around 70% of medical decisions are based on results provided by medical laboratory staff. That’s a lot of pressure on the labs to make sure their results are accurate. Clinical laboratory staff not only perform the tests used to guide diagnosis and disease prevention, but they also check all the tests they use through rigorous quality control (QC) procedures.
This involves testing samples of known values to prove that the test system and its components perform as they should and provide accurate results. To do this, laboratories require QC material. It’s important that what’s in a QC is as similar to what you’d find in a patient sample as possible. This is known as commutability. Good commutability helps limit cross-reactivity in the test and inaccurate results.
It’s also important to make sure the QC material has concentrations of analytes at similar values to those used to make diagnostic decisions. If you wanted to validate the length of the ruler on your desk, it wouldn’t be helpful to set it down on a 100m running track. Similarly, when laboratory professionals want to ensure a test is producing accurate results, they want to test the system at the critical values used to make medical decisions so that they can be confident the results at these values are accurate.
Once lab staff have confirmed the accuracy of their tests, they can begin testing patient samples. For most people, what happens to a sample after it’s taken is a bit of a mystery. MLPW is the perfect opportunity to unravel this a little:
After your sample is collected, it gets sent over to the lab. Even just moving it there needs careful handling to make sure it’s still good for testing when it arrives. Once it’s in the lab, the team checks the equipment to make sure it’s working right and giving accurate results. The QC procedure varies depending on what they’re testing for, but they always make sure their tests are legitimate. Once they’ve checked everything and carried out the tests, a pathologist looks at the results to figure out what’s going on. They use this information to help decide on the best treatment plan for you.
Even this watered-down explanation makes it sound like a lot of work, right? At Randox, we recognise the vital role and dedicated efforts of medical laboratory professionals, and the invaluable contributions they make to society, and we hope that now, you do too.
Acusera 24.7
Bingo! That’s exactly how our customers feel when they realise how much time Acusera 24.7 can save them. Our innovative and intuitive QC data software is cloud-based, allowing you to log in from anywhere in the world to review your QC data.
Along with a wide range of interactive charts, including Levey-Jennings charts, Acusera 24.7 determines measurement uncertainty and sigma metrics for you, saving you the time and stress of manually calculating these tricky statistical analyses. And that’s just the beginning. Acusera 24.7 can link to LIMS for automated data entry, meaning lab staff don’t have to manual type long datasets, unless they want to of course; we also provide both semi-automated data upload and manual data entry options.
Access to a range of reports has never been easier. Acusera 24.7 is particularly useful when gaining or renewing your accreditation, and live peer group QC data, to give additional confidence in the accuracy of your results.
But this article is supposed to be about laboratory professionals, so we won’t bang on about it anymore. We just want everyone to know about Acusera 24.7 so they can get that daily bingo! feeling for themselves. If you want to learn more about our reports, charts, advanced statistical analysis, Acusera 24.7 more generally, or how Acusera 24.7 can help you achieve your accreditation, you can follow the links to the relevant blog post.
Last year, we interviewed two of our laboratory staff, Dean and Meadhbh, to find out what a normal day looked like for them. To find out what a day in the life of a laboratory professional is like, take a look at the interviews here
If you’d like to get in touch with us to discuss the advantages of Acusera 24.7, or you’ve made up your mind and want to get in on the action, reach out to us at marketing@randox.com. We’re always happy to brag about how great Acusera 24.7 is, and how we make life simpler for more and more laboratories every day.
Lp(a) Awareness Day 2024
Novel and classical insights into Lp(a) concentration and the effects on various cardiovascular conditions.
Despite advances in understanding and technology, cardiovascular diseases (CVDs) remain a major source of mortality across the world. The World Health Organisation (WHO) estimate that 17.9 million people died due to CVDs in 2019, accounting for around 32% of deaths that year1. First described in 1963, Lipoprotein(a) (Lp(a)) is a macromolecular lipoprotein complex2 which is thought to display proatherogenic, proinflammatory3 and prothrombotic4 potential and is considered an independent causal risk factor for various types of CVD5. These properties provide several mechanisms in which elevated Lp(a) levels may contribute to CVD however the true nature of Lp(a)s relationship to CVD remains largely enigmatic.
Lp(a) concentrations in plasma are principally regulated by variation in LPA gene and levels remain relatively stable throughout one’s lifetime with lifestyle factors having little effect on their concentration6. Due to the highly heritable nature of Lp(a) concentration, those with a family history of Familial Hypercholesterolaemia (FH), elevated LDL-C levels, or Atherosclerotic cardiovascular disease (ASCVD) should be screened, their plasma Lp(a) concentration determined, and their risk of CVD established.
In the last 10 years, there have been many advances in the understanding of this ambiguous lipoprotein which support the causal association with CVD, clarify the established evidence and introduce novel mechanisms of action in relation to Lp(a), shedding light on its obscure pathophysiology. However, there are still diagnostic complications associated with Lp(a) measurement as there is little standardisation in methods of determination5.
Physiology and Genetics
Synthesised mainly in the liver, Lp(a), like LDL, is composed of a lipid centre made of cholesteryl esters and triacylglycerols, surrounded by a shell of phospholipids, free cholesterol, and an apoB-100 molecule. The major difference between other LDL molecules and Lp(a) is the presence of a polymorphic glycoprotein, apo(a), bound to apoB-100 by a single disulphide bond5. It is this apo(a) molecule which contributes to Lp(a)s pathophysiology.
Apo(a) is thought to have evolved from the plasminogen gene (PLG) around 40 million years ago and shares 78-100% sequence homology within the untranslated and coding regions of the fibrinolytic enzyme2. Like plasminogen, apo(a) contains unique domains named kringles5. While plasminogen contains 5 different kringle structures (KI to KV), apo(a) has lost KI to KIII and instead contains several forms of KIV, namely, 1 copy of KIV1 and KIV3-10, 1-40 copies of KIV2, 1 copy of KV and an inactive protein domain at the carboxyl terminus of the molecule7. These hydrophilic subunits are highly polymorphic due to the variation in KIV2 repeats. Individuals may possess two different isoforms of apo(a) one of which will have been passed down from each parent and are expressed codominantly2. These isoforms are dependent on the number of KIV2 repeats they contain2. Isoforms with less KIV2 repeats produce smaller apo(a) isoforms which are found at a higher concentration compared with larger isoforms8 due to the increased rate at which the smaller molecules can be synthesised5. The polymorphisms in KIV2 repeats account for up to 70% of the variation seen in concentration between individuals, with the remainder being attributed to differences in protein folding, transport, and single nucleotide polymorphisms (SNPs)5. SNPs are central in the heterogeneity of apo(a), effecting RNA splicing, nonsense mutations and 5’ untranslated region of the LPA gene resulting in shorter gene translation5,8.

Lp(a) Pathophysiology
Lp(a) is thought to contribute to the risk of CVD through multiple mechanisms. Firstly, Lp(a) molecules display all the same atherosclerotic risk as LDL-C molecules due to their similar fundamental composition, for example, their propensity for oxidisation upon entering the vessel wall, and promotion of atherosclerosis through inflammatory and immunogenic mechanisms 9. However, Lp(a) displays more proatherogenic potential due to the presence of the apo(a) molecule. The structure of apo(a) results in decreased fibrinolysis. Due to its structural similarities, apo(a) competes with plasminogen for binding sites, competitively inhibiting plasminogen, ultimately resulting in reduced fibrinolysis9.
Lp(a) is thought to be a preferential carrier of oxidised phospholipids2 (OxPLs) which covalently bind to apo(a), increase expression of inflammatory proteins, and stimulate the secretion of IL-8 and monocyte chemoattractant protein-1, enhancing its ability to cross the vessel wall9. Some claims require further investigation, however, studies have been carried out which show inhibition of plasminogen activation in the presence of Lp(a)10. It is this indirect mechanism that Lp(a) is thought to conduct its prothrombotic activity8,9.
Clinical Evidence
Many studies have been carried out to determine the association of Lp(a) concentration and CVD risk. Studies such as the Copenhagen General Population Study, the Copenhagen City Heart Study, Dallas Heart Study, and Ischemic Heart Disease Studies provide strong evidence for Lp(a) as a causal risk factor for CVD. Data analysis of the Copenhagen General Population Study reveal that 20% of subjects displayed Lp(a) concentrations of more than 42mg/dl, or around 105nmol/L11, which is considered to result in increased risk of atherosclerotic disease5. It is important to note, there is no accepted conversion factor for converting Lp(a) concentration from mg/dl to nmol/L due to the variability of apo(a) kringles. The unitage will depend on the assay method used5. Another study in a healthcare organisation in Israel showed that Myocardial Infarction (MI) and Coronary Artery Disease was 2.5 times more common in those with high levels of Lp(a) than in the age and sex matched control group3. This study3, along with others5,6,12 describes a linear relationship between Lp(a) concentration and CVD risk, showing at least a 3-fold increase in ASCVD and MI events in adults with Lp(a) concentrations in the top 1% when compared with those in the with concentrations in the bottom 20%3.
The major variation in Lp(a) concentration seen throughout the population, is further evident between ethnicities and sexes. On average, Caucasian subjects display the lowest Lp(a) concentrations, with Black subjects displaying the highest concentrations5. However, the large number of functional variants are consistent across ethnicities suggesting that it is the KIV2 repeats and SNPs that are the major factors contributing to Lp(a) concentration regardless of ethnicity. Lp(a) concentrations are higher in women than men8 with levels increasing post-menopause thought to be caused by a decrease in oestrogen3.
Lp(a) Testing and Screening
The European Atherosclerosis Society (EAS) recommend that all adults are tested at least once in their lifetime to identify individuals who have high levels of Lp(a) and therefore high CVD risk. Screening is also recommended in children who have a family history of Ischaemic stroke, premature ASCVD or high Lp(a) levels in the absence of other identifiable risk factors8. Testing has been associated with reduced mortality rates. This is thought to be because of increased and intensified therapy for those who are identified as high risk due to high plasma Lp(a) concentration6.
There are various assays available for the determination of Lp(a) concentration which vary in accuracy and precision. Many of these assays utilise polyclonal antibodies which recognise different antigenic determinants8. Due to the variability in apo(a) structure and KIV repeats, these assays often overestimate the concentration of large isoforms and underestimate concentration of small isoforms when determining the true Lp(a) levels9. This variation can be partially nullified by using a calibrator series and by selecting a method which is traceable to WHO/IFCC reference material. This allows laboratories to confidently identify individuals considered high risk but may still prove problematic when patients’ results report closer to the assay thresholds.
One study13 compared 5 commercially available Lp(a) assays on an automated clinical chemistry analyser. The assays tested were manufactured by Diazyme, Kamiya, MedTest, Roche, and Randox. The authors show that all the assays tested met the manufacturers claims for sensitivity, linearity, and precision. However, significant bias was observed in 4 out of 5 assays. The only assay which did not display significant bias was the Randox Lp(a) Assay which is traceable to WHO/IFCC reference material. This report highlights the importance of measuring and reporting Lp(a) in molar concentration rather than in mass units to facilitate standardisation and harmonisation in Lp(a) testing13.
Current and Emerging Therapies
Statins are one of the most potent treatments for the primary prevention of ASCVD through the reduction of LDL-C concentration. However, recent studies reveal that statins have no effect on Lp(a) concentration3 and others suggest that statin administration can increase Lp(a) concentration by up to 11%5,9. Nonetheless, EAS do not recommend statin therapy be halted as their strong ameliorative effects on CVD risk are well established and surmount the risk related to increased Lp(a) concentration8.
Niacin (Nicotinic acid) is another established treatment for the reduction of CVD events and act by increasing HDL levels. Niacin can reduce Lp(a) concentration though the reduction of gene expression in a dose-dependent manner5. However, Niacin therapy has not been proven to have beneficial effects on CVD risk8.
A recent metanalysis showed a 26% reduction in serum Lp(a) concentration through treatment with PCSK9 inhibitors. This is thought to be due to a shortage of apoB-100 molecules either because of reduced synthesis or competitive binding with other LDL receptors, resulting in reduced Lp(a) concentration5. Several studies show the efficacy of PCSK9 inhibitors in reducing CVD risk, but this is not yet an approved therapy5,8.
New therapeutic strategies aim to target hepatocytes, the site of apo(a) synthesis, to reduce Lp(a) concentration. Antisense Oligonucleotides (ASOs) inhibit apo(a) mRNA in the nucleus and cytoplasm, ultimately inhibiting Lp(a) secretion5 through the cleavage of the sense strand by ribonuclease H19. While still in clinical trials, ASO therapies show promise in the battle to reduce CVD risk with some studies displaying an overall reduction in Lp(a) concentration of more than 80%9.
Conclusions
There have been major advances in the understanding of Lp(a) pathophysiology in the last 10 years establishing this macromolecular complex as an independent causal risk factor for various forms of CVD, however, more investigation is required to fully understand the mechanisms responsible for this association. With many national healthcare organisations and the EAS recommending universal testing for Lp(a) in adults, more emphasis should be placed on raising awareness of the importance of Lp(a) screening. Finally, more research is needed into therapies which succeed at lowering Lp(a) concentration. While some therapies are in clinical trials, there are currently no approved therapies that achieve this goal.
The Randox Lp(a) assay is calibrated in nmol/L, traceable to the WHO/IFCC reference material, and displays an excellent correlation coefficient of r=0.995 with when compared with other commercially available methods. To accompany this liquid ready-to-use reagent we also offer a dedicated 5 point calibrator with accuracy-based assigned target values (in nmol/l) is available, accurately reflecting the heterogeneity of the apo(a) isoforms.
For more information on this revolutionary assay, visit randox.com/lipoproteina/ or reach out to us at marketing@randox.com.
References
- World Health Organization. Cardiovascular Diseases. World Health Organization. Published June 11, 2021. https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)
- Schmidt K, Noureen A, Kronenberg F, Utermann G. Structure, function, and genetics of lipoprotein (a). Journal of Lipid Research. 2016;57(8):1339-1359. doi:https://doi.org/10.1194/jlr.r067314
- Zafrir B, Aker A, Saliba W. Extreme lipoprotein(a) in clinical practice: A cross sectional study. International Journal of Cardiology Cardiovascular Risk and Prevention. 2023;16:200173. doi:https://doi.org/10.1016/j.ijcrp.2023.200173
- Pino BD, Gorini F, Gaggini M, Landi P, Pingitore A, Vassalle C. Lipoprotein(a), Cardiovascular Events and Sex Differences: A Single Cardiological Unit Experience. Journal of Clinical Medicine. 2023;12(3):764. doi:https://doi.org/10.3390/jcm12030764
- Stürzebecher PE, Schorr JJ, Klebs SHG, Laufs U. Trends and consequences of lipoprotein(a) testing: Cross-sectional and longitudinal health insurance claims database analyses. Atherosclerosis. 2023;367:24-33. doi:https://doi.org/10.1016/j.atherosclerosis.2023.01.014
- Lampsas S, Xenou M, Oikonomou E, et al. Lipoprotein(a) in Atherosclerotic Diseases: From Pathophysiology to Diagnosis and Treatment. Molecules. 2023;28(3):969. doi:https://doi.org/10.3390/molecules28030969
- Vuorio A, Watts GF, Schneider WJ, Tsimikas S, Kovanen PT. Familial hypercholesterolemia and elevated lipoprotein(a): double heritable risk and new therapeutic opportunities. Journal of Internal Medicine. 2019;287(1):2-18. doi:https://doi.org/10.1111/joim.12981
- Kronenberg F, Mora S, Stroes ESG, et al. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: a European Atherosclerosis Society consensus statement. European Heart Journal. 2022;43(39):3925-3946. doi:https://doi.org/10.1093/eurheartj/ehac361
- Tsimikas S. A Test in Context: Lipoprotein(a): Diagnosis, Prognosis, Controversies, and Emerging Therapies. Journal of the American College of Cardiology. 2017;69(6):692-711. doi:https://doi.org/10.1016/j.jacc.2016.11.042
- Boffa MB, Koschinsky ML. Lipoprotein (a): truly a direct prothrombotic factor in cardiovascular disease? Journal of Lipid Research. 2016;57(5):745-757. doi:https://doi.org/10.1194/jlr.r060582
- Enkhmaa B, Anuurad E, Berglund L. Lipoprotein (a): impact by ethnicity and environmental and medical conditions. Journal of Lipid Research. 2016;57(7):1111-1125. doi:https://doi.org/10.1194/jlr.r051904
- Svilaas T, Klemsdal TO, Bogsrud MP, et al. High levels of lipoprotein(a) – assessment and treatment. Tidsskrift for Den norske legeforening. Published online January 12, 2023. doi:https://doi.org/10.4045/tidsskr.21.0800
- Wyness SP, Genzen JR. Performance evaluation of five lipoprotein(a) immunoassays on the Roche cobas c501 chemistry analyzer. Practical Laboratory Medicine. 2021;25:e00218. doi:https://doi.org/10.1016/j.plabm.2021.e00218
World Tuberculosis Day 2024
Tuberculosis in Brief
When we think of Tuberculosis (TB) we tend to think of an old-timey disease. Doc Holliday, the famous gunslinger, died of consumption, the old-world name for TB. As did Fantine from Victor Hugo’s “Les Misérables” and Nicole Kidman’s, Santine, in the 2001 movie, Moulin Rouge! For the videogame fans out there, you might be familiar with Arthur Morgan from Red Dead Redemption 2 who, depending on how you played the game, may have suffered a similar fate. However, this disease is still prevalent around the world today. TB is a bacterial infection caused by Mycobacterium tuberculosis estimated to infect around 10 million people and is responsible for up to 1.5 million deaths each year1.
Originally discovered in 1882, M. tuberculosis is an airborne pathogen which primarily affects the lungs but can also affect other parts of the body2. TB infection exists in 3 states: latent, subclinical, and active. A latent TB infection is asymptomatic and non-transmissible. Subclinical infections are also asymptomatic but transmissible and will produce a positive culture. Finally, active disease is a transmissible state associated with the symptoms of TB2. The World Health Organization (WHO) estimates that around ¼ of the world population is infected with M. tuberculosis3. Up to 15% of those infected with TB will progress to active disease, while those who do not are at a heightened risk of infection throughout the rest of their lives4. Compared with some other bacterial diseases, TB is not particularly infectious. An infected individual is estimated to infect between 3-10 people per year2. However, subclinical TB infections present a challenge in reducing transmission because asymptomatic individuals may unknowingly spread the disease – over 1/3 of TB infections are never formally diagnosed5.
The symptoms of an active TB infection include fever, fatigue, lack of appetite, weight loss, and where the infection effects the lungs, a persistent cough and haemoptysis (coughing up blood). HIV-infection is a major risk factor for TB infection and mortality. Up to 12% of all new cases and 25% of TB deaths occur in HIV-positive persons2. Other risk factors for the development of TB are, malnutrition, poor indoor air quality, Type 2 diabetes, excessive alcohol consumption and smoking1.
TB is present around the world. However, as you might expect from the risk factors, low-to-middle income and developing countries account for a disproportionate number of cases. According to WHO, half of all TB infections are found in 8 countries: Bangladesh, China, India, Indonesia, Nigeria, Pakistan, Philippines, and South Africa.
Without effective treatment, TB will kill and estimated 50% of those infected2. Treatment for TB typically involves first-line antibiotics such as isoniazid, rifampicin, pyrazinamide, and ethambutol, with second-line drugs including fluoroquinolones and injectable aminoglycosides6. Nonetheless, drug-resistant TB accounts for an inordinately large amount of the global AMR burden which can arise from both transmitted and acquired resistance. Resistant M. tuberculosis strains are classified as monoresistant – those resistant to 1 drug; multi-drug resistant (MDR) – those resistant to 2 or more first line treatments, commonly isoniazid and rifampicin; and extensively drug resistant (XDR) – MDR strains which are also resistant to second line therapies like fluoroquinolones and aminoglycosides6.
Global rates of TB have been declining. An estimated 75 million lives have been saved since 20001. Furthermore, between 2015 and 2020, TB incidence fell by 13.5%7. However, the progress made over the last decade has been compromised by the COVID-19 pandemic, illustrated by a, 18% drop in diagnosis between 2019 and 20207. Explanations for this decline include delayed treatment because of lack of access to public transport and healthcare facilities, disruption of laboratory services, a personal desire to avoid the stigma of disease and misdiagnosis due to the similarities in symptoms between TB and COVID-19.
The theme for World Tuberculosis Day 2024 is “Yes, we can end TB!” The WHO have set targets of an 80% decline in new cases and a 90% drop in TB-related deaths by 2030. Screening and preventative treatments are crucial to achieving these goals. Therefore, novel methods of detection which are quick, inexpensive and include drug resistance identification are needed.
Mycobacterium Tuberculosis EQA
It is important for those carrying out TB testing to ensure their instruments and methods are accurate and effective. External Quality Assessment (EQA) programmes are an essential part of this process. QCMD is an independent international EQA organisation primarily focused on molecular infectious diseases to over 2000 participants in over 100 countries.
QCMD offers 2 programmes for those testing for TB through molecular methods: Mycobacterium tuberculosis DNA and Mycobacterium Tuberculosis Drug Resistance.
Mycobacterium tuberculosis DNA EQA Programme
Mycobacterium Tuberculosis Drug Resistance EQA Programme
Mycobacterium Tuberculosis Quality Controls
Those conducting research into TB infections and new methods of detection, screening and drug resistance profiling need to be confident that the equipment they are using is up to the task. Qnostics is a leading provider of Quality Control solutions for molecular infectious disease testing. Our range comprises hundreds of characterised viral, bacterial, and fungal targets covering a wide range of diseases.
Q Controls
Our range of positive run, whole pathogen, third party controls are designed to monitor assay performance on a routine basis. As true third-party controls, assay drift is detected, monitored, and managed, helping to ensure accurate and reliable results. The use of third-party controls will also help to support ISO 15189:2012 regulatory requirements.
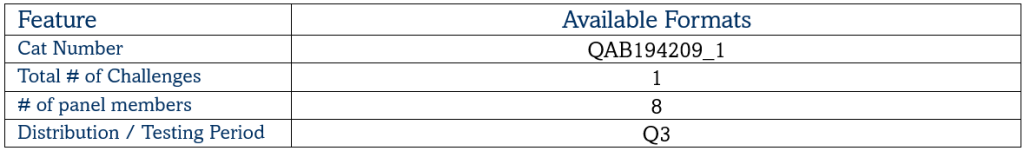
Mycobacterium tuberculosis (MTB) Q Control 01
Target Pathogen – Mycobacterium tuberculosis (MTB)
Matrix – Synthetic Sputum
Stability – Single use control designed to be used immediately minimising the risk of contamination
Shelf Life – Up to 2 years from date of manufacture
Regulatory Status – Research Use Only

Mycobacterium tuberculosis (MTB) Rifampicin Resistant Q Control
Compatible for use with Cepheid analysers, this whole pathogen positive control is designed to monitor the performance of molecular assays used in the detection of Rifampicin resistant Mycobacterium tuberculosis.
Target Pathogen – Mycobacterium tuberculosis (MTB)
Target Genotype – Rifampicin Resistance
Matrix – Synthetic Sputum
Stability – Single use control designed to be used immediately minimising the risk of contamination
Shelf Life – Up to 2 years from date of manufacture
Regulatory Status – Research Use Only

Mycobacterium tuberculosis (MTB) Evaluation Panel Control
QNOSTICS Evaluation Panels cover a range of genotypes and/or levels, and may be used to evaluate assay characteristics, confirm performance claims, and ultimately ensure the assay is fit for purpose. Evaluation Panels may also be used in the validation of clinical assays and the development of new diagnostic tests.
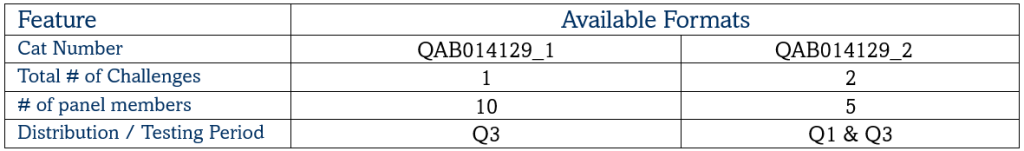
This dedicated MTB Evaluation Panel comprises 3 targets relating to Mycobacterium tuberculosis for validating a new assay or instrument to ensure that everything is working as expected. High and medium concentrations are provided alongside a negative sample.
Target Pathogens – MTB, M. bovis, Rifampicin (Rif) resistant MTB, Isoniazid (INH) resistant MTB, Negative
Matrix – Synthetic Sputum
Panel Members – 8 (Including a negative)
Stability – Single use. Once thawed, use immediately
Shelf Life – up to 2 years from date of manufacture
Regulatory Status – Research Use Only

If you are interested in any of the TB quality control products shown above, or any other products from our wide catalogue of molecular controls and EQA programmes, get in touch with us today at marketing@randox.com. To learn more, see the links below which will take you to the relevant sites and brochures.
QNOSTICS – www.randox.com/molecular-infectious-disease-controls/
QCMD – https://www.qcmd.org/
References
- World Health Organisation. Tuberculosis. Fact Sheets. Published November 7, 2023. Accessed March 21, 2024. https://www.who.int/news-room/fact-sheets/detail/tuberculosis
- Pai M, Behr MA, Dowdy D, et al. Tuberculosis. Nat Rev Dis Primers. 2016;2(1):16076. doi:10.1038/nrdp.2016.76
- World Health Organisation. Tuberculosis. https://www.who.int/health-topics/tuberculosis.
- Andrews JR, Noubary F, Walensky RP, Cerda R, Losina E, Horsburgh CR. Risk of Progression to Active Tuberculosis Following Reinfection With Mycobacterium tuberculosis. Clinical Infectious Diseases. 2012;54(6):784-791. doi:10.1093/cid/cir951
- Adigun R, Singh R. Tuberculosis. StatPearls Publishing; 2024.
- Liebenberg D, Gordhan BG, Kana BD. Drug resistant tuberculosis: Implications for transmission, diagnosis, and disease management. Front Cell Infect Microbiol. 2022;12. doi:10.3389/fcimb.2022.943545
- World Health Organisation. Global Tuberculosis Report 2022.; 2022. Accessed March 21, 2024. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022
A Peculiar Problem in Pregnancy and the Placenta
Complications and Diagnosis of Pre-eclampsia
When we consider our most important organ its intuitive to choose the heart, the lungs or even the kidneys. However, there’s another without which none of us would be here to have the discussion. This ephemeral organ provides us with the nutrients necessary for development, removes malevolent agents, provides our initial immunity and much more, before being cast off as we enter the world. We are, of course, talking about the placenta. Indeed, all our organs work together to support life and it’s arbitrary to imbue one with more importance than the others. Nevertheless, as our first organ, the significance of the placenta is irrefutable.
Placental dysfunction, along with several other factors, is known to contribute to the development of pre-eclampsia – a complex, multisystem hypertensive disorder of pregnancy. While the aetiology of pre-eclampsia remains largely unknown, the grave complications associated with it have driven development of novel methods for predicting its onset.
Pre-eclampsia and Epidemiology
Pre-eclampsia is traditionally defined as new onset hypertension and proteinuria in pregnancy1, however, the International Federation of Gynaecology and Obstetrics’ (FIGO) clinical definition describes it as sudden onset hypertension (>20 weeks of gestation) and at least one of the following: proteinuria, maternal organ dysfunction or uteroplacental dysfunction2. It is responsible for an estimated 70’000 maternal deaths, and 500’000 foetal deaths globally3. Pre-eclampsia affects around 4% of pregnancies in the US and is more common in low-to-middle income countries (LMICs), displaying an overall pooled incidence of 13% in a cohort from sub-Saharan Africa4. The risk factors for pre-eclampsia are shown in the graphic below.

Pre-eclampsia is associated with increased morbidity and mortality worldwide. In the US, pre-eclampsia is the foremost cause of maternal death, severe maternal morbidity, maternal intensive care admissions and prematurity5.
Classical classification of pre-eclampsia included early-onset (<34 weeks gestation) and late-onset (>34 weeks gestation). However, this classification lacks clinical utility as it does not accurately illustrate maternal or foetal prognosis. Therefore, the International Society for the study of Hypertension in Pregnancy (ISSHP) and contemporary studies prefer to classify pre-eclampsia as preterm (delivery <37 weeks of gestation), term (delivery ≥37 weeks of gestation) and postpartum pre-eclampsia (after delivery).

Complications
Pre-eclampsia has been associated with acute and chronic complications for both mother and child. Worldwide risk of maternal and foetal morbidity displays adjusted odds ratios of 3.73 and 3.12, respectively (pre-eclampsia vs non pre-eclampsia)6.
Acute Maternal Complications
A range of neurological complications are associated with pre-eclampsia. The most obvious is eclampsia, defined as seizures in pregnant women commonly from 20 weeks of gestation or after birth7. Eclampsia has two proposed mechanisms: abnormal placentation reduces blood supply and causes oxidative stress, leading to endothelial damage; and elevated blood pressure in pre-eclampsia disrupts cerebral vasculature, causing hypoperfusion and damage8. In high-income countries (HICs), most women make a full recovery, however, more severe cases of eclampsia can result in permanent disability or brain damage7.
Stroke is a significant complication of pre-eclampsia, constituting 36% of strokes related to pregnancy9. The hypertension characteristic of pre-eclampsia can weaken the walls of blood vessels causing subarachnoid or intracerebral haemorrhage resulting in haemorrhagic stroke. Ischaemic stroke is also of concern due to blood clotting complications which will be discussed later.
Additonal neurological complications include visual scotoma, cortical blindness, cerebral venous sinus thrombosis, cerebral vasoconstriction syndrome and posterior reversible encephalopathic syndrome (PRES). Notably, the last three in this list frequently manifest postpartum without warning6.
HELLP (Haemolysis, Elevated Liver enzymes and Low Platelets) syndrome is a liver and blood clotting disorder and life-threatening complication of pre-eclampsia. HELLP syndrome most commonly presents immediately postpartum but can manifest any time after 20 weeks of gestation7. Microangiopathy, or small blood vessel disorder, leads to ischaemia and a subsequent increase in oxidative stress and inflammation, causing an increase in liver enzymes and participates in the initiation of HELLP. Thrombocytopenia, or platelet deficiency, is considered a product of platelet depletion resulting from heightened platelet activation triggered by widespread endothelial damage6.
Another blood clotting condition associated with pre-eclampsia is Disseminated intravascular coagulation (DIC)7, described as the dysfunction of the maternal blood clotting system resulting in multiple organ dysfunction syndrome10. DIC can cause excessive bleeding due to lack of clotting proteins, or the formation of clots due to overactive clotting proteins, ultimately causing organ damage10.
As described earlier, proteinuria is included in the diagnostic criteria for pre-eclampsia, suggesting involvement of the kidneys. This is caused by high concentrations of soluble FMS like Tyrosine kinase 1 (sFLT-1), a placental angiogenic factor, which inhibits proteins of the podocyte slit diaphragm6; the machinery involved in preventing the leakage of proteins into the urine11. Reduced levels of Vascular Endothelial Growth Factor (VEGF) and Placental Growth Factor (PlGF) stimulates Endothelin 1 expression6, known to promote podocyte detachment, further contributing to proteinuria12.
Finally, Pulmonary oedema, excessive fluid accumulation in the lungs, is an acute and life-threatening complication associated with pre-eclampsia, the likelihood of which is increased via administration of antihypertensive medications6.
Acute Neonatal Complications
There are several documented complications affecting the baby of a pre-eclamptic mother. Firstly, Intrauterine growth restriction (IUGR) can result in underdevelopment of the foetus because of deficient transfer of oxygen and other nutrients from mother to child13. This can result in low birth weight, particularly when pre-eclampsia occurs prior to 37 weeks of gestation7. In pre-eclampsia with severe symptoms, delivery frequently occurs prematurely, either spontaneously or through induction. Preterm delivery can result in complications such as neonatal respiratory distress syndrome and neonates often require ICU admission7. Additionally, there is increased risk of stillbirth in pre-eclamptic pregnancies with relative risk shown to be 1.45 (95% Cl 1.20-1.76)14. Other complications documented in neonates born through pre-eclamptic pregnancies include neonatal thrombocytopenia, bronchopulmonary dysplasia, and a range of neurodevelopment outcomes15.
Long-term Complications
The only known cure for pre-eclampsia is delivery. However, the complications for both mother and child can last long after even an uncomplicated delivery. After a pre-eclamptic pregnancy, women are increased risk of end stage renal disease (4.7-fold), stroke (4-fold) and vascular dementia (3-fold) later in life5. Women are also at increased risk of other cardiovascular disease (CVD) including chronic hypertension, coronary artery disease, congestive heart failure5, and ischaemic heart disease13. In offspring, IUGR increases the risk of development of hypertension and other CVD13. Finally, offspring have been shown to be at higher risk of increased body mass index, changes in neuroanatomy, reductions in cognitive function, and hormonal abnormalities13.

sFLT-1/PlGF ratio
The pathophysiology of pre-eclampsia is complex and enigmatic. However, placental dysfunction is known to be a factor in pre-eclampsia development. The placental-related angiogenic factors, sFLT-1 (anti-angiogenic) and PlGF (pro-angiogenic), have been implicated in this development. This ratio provides a useful measure of placental dysfunction as a sharp increase in sFLT-1 and decrease in PlGF has been shown approximately 5 weeks before onset of pre-eclampsia16.
Until recently, diagnosis of pre-eclampsia was one of clinical manifestation. However, studies such as PROGNOSIS17 and PROGNOSIS Asia18, along with others19,20, have shown strong utility of this ratio. The PROGNOSIS study showed that a ratio cutoff of ≥38 was useful for ruling out pre-eclampsia within 1 week with a negative predictive value (NPV) of 99.3% or 4 weeks with a positive predictive value (PPV) of 36.7%17. The definitions of pre-eclampsia used by ICCHP and American College of Obstetricians and Gynaecologists (ACOG) have a PPV of around 20%, but when used in combination with the sFLT-1/PlGF ratio, the PPV is enhanced to 65.5% for ruling in pre-eclampsia within 4 weeks.21.
Similar results have been shown in an Asian cohort in the PROGNOSIS Asia Study. Using the same cutoff value, this study reported an NPV of 98.9%18. Furthermore, in a sub analysis of this cohort that looked at Japanese participants, a cutoff of ≥38 displayed an NPV of 100% for ruling out pre-eclampsia within 1 week and a PPV of 32.4% for ruling in within 4 weeks22.
Accurate Identification is Essential
Like all clinical assays, those used to determine the sFLT-1/PlGF ratio are subject to rigorous quality control, essential to ensure accurate results and diagnosis. The complications of pre-eclampsia are severe and often life-threating for both mother and child. Early and accurate identification is imperative for optimal monitoring, management, and timely interventions to reduce the risk of the grave consequences associated with pre-eclampsia.
The utility of the sFLT-1/PlGF ratio has been shown over various large cohorts and provides improved identification when used in combination with established clinical definitions. While the enigma of pre-eclampsia persists, the dedication of the scientific community to unravel its complexities ensures a future where expectant mothers may benefit from more effective and tailored strategies to mitigate the risks associated with this puzzling condition. Continued research endeavours will undoubtedly shape the landscape of maternal-foetal medicine, fostering advancements that hold the promise of improved outcomes for both mothers and their unborn children.
At Randox Quality Control, we’ve introduced our Pre-eclampsia Control to the Acusera IQC range for use with in vitro diagnostic assays for the quantitative determination of PlGF and sFlt-1 in human serum and plasma.
Our true third-party Pre-eclampsia control comes with clinically relevant, assayed target values, is liquid-frozen for user convenience, utilises a human-based, commutable matrix, and has a 30-day open vial stability.
For more information on this, or any of our other controls, browse our brochure, or reach out to us today at marketing@randox.com for more information.
References
- American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in Pregnancy. Obstetrics & Gynecology. 2013;122(5):1122-1131. doi:10.1097/01.AOG.0000437382.03963.88
- Poon LC, Shennan A, Hyett JA, et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre‐eclampsia: A pragmatic guide for first‐trimester screening and prevention. International Journal of Gynecology & Obstetrics. 2019;145(S1):1-33. doi:10.1002/ijgo.12802
- Karrar SA, Hong PL. Preeclampsia. StatPearls Publishing; 2023.
- Jikamo B, Adefris M, Azale T, Alemu K. Incidence, trends and risk factors of preeclampsia in sub-Saharan Africa: a systematic review and meta-analysis. PAMJ – One Health. 2023;11. doi:10.11604/pamj-oh.2023.11.1.39297
- Rana S, Lemoine E, Granger JP, Karumanchi SA. Preeclampsia. Circ Res. 2019;124(7):1094-1112. doi:10.1161/CIRCRESAHA.118.313276
- Dimitriadis E, Rolnik DL, Zhou W, et al. Pre-eclampsia. Nat Rev Dis Primers. 2023;9(1):8. doi:10.1038/s41572-023-00417-6
- NHS. Pre-eclampsia. Health A to Z. Published September 28, 2021. Accessed January 3, 2024. https://www.nhs.uk/conditions/pre-eclampsia/complications/
- Magley M, Hinson MR. Eclampsia. StatPearls Publishing; 2023.
- Crovetto F, Somigliana E, Peguero A, Figueras F. Stroke during pregnancy and pre-eclampsia. Curr Opin Obstet Gynecol. 2013;25(6):425-432. doi:10.1097/GCO.0000000000000024
- Costello RA, Nehring SM. Disseminated Intravascular Coagulation. StatPearls Publishing; 2023.
- Kawachi H, Fukusumi Y. New insight into podocyte slit diaphragm, a therapeutic target of proteinuria. Clin Exp Nephrol. 2020;24(3):193-204. doi:10.1007/s10157-020-01854-3
- Trimarchi H. Mechanisms of Podocyte Detachment, Podocyturia, and Risk of Progression of Glomerulopathies. Kidney Dis (Basel). 2020;6(5):324-329. doi:10.1159/000507997
- Turbeville HR, Sasser JM. Preeclampsia beyond pregnancy: long-term consequences for mother and child. American Journal of Physiology-Renal Physiology. 2020;318(6):F1315-F1326. doi:10.1152/ajprenal.00071.2020
- Harmon QE, Huang L, Umbach DM, et al. Risk of Fetal Death With Preeclampsia. Obstetrics & Gynecology. 2015;125(3):628-635. doi:10.1097/AOG.0000000000000696
- Backes CH, Markham K, Moorehead P, Cordero L, Nankervis CA, Giannone PJ. Maternal Preeclampsia and Neonatal Outcomes. J Pregnancy. 2011;2011:1-7. doi:10.1155/2011/214365
- Verlohren S, Galindo A, Schlembach D, et al. An automated method for the determination of the sFlt-1/PIGF ratio in the assessment of preeclampsia. Am J Obstet Gynecol. 2010;202(2):161.e1-161.e11. doi:10.1016/j.ajog.2009.09.016
- Zeisler H, Llurba E, Chantraine F, et al. Predictive Value of the sFlt-1:PlGF Ratio in Women with Suspected Preeclampsia. New England Journal of Medicine. 2016;374(1):13-22. doi:10.1056/NEJMoa1414838
- Bian X, Biswas A, Huang X, et al. Short-Term Prediction of Adverse Outcomes Using the sFlt-1 (Soluble fms-Like Tyrosine Kinase 1)/PlGF (Placental Growth Factor) Ratio in Asian Women With Suspected Preeclampsia. Hypertension. 2019;74(1):164-172. doi:10.1161/HYPERTENSIONAHA.119.12760
- Hughes RCE, Phillips I, Florkowski CM, Gullam J. The predictive value of the sFlt‐1/PlGF ratio in suspected preeclampsia in a New Zealand population: A prospective cohort study. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2023;63(1):34-41. doi:10.1111/ajo.13549
- Nikuei P, Rajaei M, Roozbeh N, et al. Diagnostic accuracy of sFlt1/PlGF ratio as a marker for preeclampsia. BMC Pregnancy Childbirth. 2020;20(1):80. doi:10.1186/s12884-020-2744-2
- Verlohren S, Brennecke SP, Galindo A, et al. Clinical interpretation and implementation of the sFlt-1/PlGF ratio in the prediction, diagnosis and management of preeclampsia. Pregnancy Hypertens. 2022;27:42-50. doi:10.1016/j.preghy.2021.12.003
- Ohkuchi A, Saito S, Yamamoto T, et al. Short-term prediction of preeclampsia using the sFlt-1/PlGF ratio: a subanalysis of pregnant Japanese women from the PROGNOSIS Asia study. Hypertension Research. 2021;44(7):813-821. doi:10.1038/s41440-021-00629-x
International Day of Women and Girls in Science 2024
For the 9th consecutive year, the field of science has taken this day to celebrate women in the STEM industries and their achievements. The representation of women in STEM is climbing, however it remains low, with estimates claiming women make up only around 26% of the workforce. By celebrating the accomplishments of women in these fields, we hope to encourage more girls to enter the world of science and engineering and challenge the adversity women in STEM all too often face.
In honour of International Day of Women and Girls in Science 2024, we’ve looked at some of the most important achievements in the life sciences. Some of the names you’ll be familiar with, others may be new. We’ll travel to Ancient Greece where we will learn about the first female science writer and surgeon, before coming back to today to recognise some of the most groundbreaking innovations in medicine. For far too long the door to a career in the life sciences has been all but closed for women, as you will discover in this article. Yet some of the discoveries and triumphs over adversity we’ll look at are arguably some of the most important achieved by humanity.
Metrodora
In Ancient Greece, it was believed that science was derived directly from the gods. This meant, like other divine disciplines, women were not allowed to practice medicine. However, such a technicality did not stop our first heroine of science. Before her exploits in Greece, Metrodora was likely born and educated in Egypt where men and woman were equals, unlike much of the ancient world. In fact, some believe Metrodora to be an alias of the famous Cleopatra VII, Queen of Egypt. There is some debate about when Metrodora lived; some say between 200-400 CE, others claim it was more likely to be during the 7th century CE. The name Metrodora is particularly fitting for a woman of her accolades. In Greek, metro can be translated as womb, and dora means gift.
Metrodora was the author of a textbook, making her the first female science writer, spanning 2 volumes and 108 chapters entitled On the Disease and Cures of Women, in which she describes in detail her theories and findings on the topics of female health and the reproductive system. She is thought to have devised treatments for sterility, infections of the female reproductive system, menorrhagia (heavy periods) as well as a method for determining sexual abuse in women. Not to be limited by writing and medicine, Metrodora was also a surgeon, cited as removing dead embryos to save the lives of mothers who miscarried, removing cancers of the breast and uterus and was even among the first to perform cosmetic surgery. Metrodora reconciled this with her Hippocratic duty to help women who had been abused through aesthetic facial and breast reconstruction and the restructuring of the hymen of women who had suffered this fate.
Her pioneering work, however, was quite nearly forgotten forever as she was largely overlooked by her contemporaries. But thankfully, some of her texts are preserved in the Laurentian Library in Florence. Metrodora’s commitment to medicine and female health is summed up perfectly in the opening words of her text, “some of them are intricate to treat and others are fatal, by these notes we will recognise each one”.

Elizabeth Blackwell (1821-1910)
Elizabeth Blackwell was always destined to shake the status quo. Her father, Samuel, was a Quaker and an antislavery activist. Among her siblings are Henry, an abolitionist and women’s suffrage supporter; her sister, Emily, who followed Elizabeth into medicine; and her sister-in-law, Antoinette Brown Blackwell, who was the first female minister in mainstream Protestantism.
She was inspired into a life of medicine after a close friend had confessed embarrassment of her treatment by male doctors and suggested she’d have been more comfortable if attended to by a female physician. After a series of rejections from numerous medical schools, Elizabeth was finally accepted to Geneva College, New York. However, this acceptance was intended as a practical joke. Not to be discouraged, Elizabeth proved them all wrong, graduating top of her class in 1849 and becoming the first woman to graduate medical school. Dr Blackwell then practiced in London and Paris and was one of the first advocates for the importance of hygiene in medicine, noting that male doctors frequently failed to wash their hands, which led to the spread of epidemics.
In 1851, Elizabeth made the trip back to New York where discrimination was still rife. Once again, her persistence led to the opening of the New York Infirmary for Women and Children in 1857 with Dr Emily Blackwell and Dr Marie Zakrzewska. This institution was a haven for women who needed medical treatment but were often too poor to afford it, and for female physicians struggling to get work in the field. Among her laurels, she played a role in the inception of the National Health Society, established in 1871, which aimed to spread knowledge of public health, and is considered the predecessor to the National Health Service.

Marie Curie (1867-1934)
Marie Curie is among the most famous of the women in science, so we won’t spend too much time on her life and accomplishments here. However, its impossible to have the discussion without mentioning her invaluable contribution to science. In often poor laboratory conditions with worse equipment, she, and her husband Pierre Curie, made some pivotal discoveries including the isolation of polonium and radium. Marie Curie developed techniques to separate radium from radioactive residues which allowed it to be studied extensively and eventually, its use as a therapeutic agent.
In 1903, Marie and Pierre Curie were awarded half of the Nobel Prize for Physics for their work on spontaneous radiation. Then, in 1911, Marie Curie was given a second Nobel Prize, this one for chemistry, for her work on radioactivity. In recognition of her groundbreaking work leading to novel cancer therapies, the charity Marie Curie was named in her honour, immortalising her and her contributions to the field.

Gerty Cori (1896-1957)
Here we find another woman whose name outshines that of her husband, Carl, with whom she collaborated for most of her scientific career. Gerty graduated from medical school in 1920, along with her husband, before they emigrated to America in 1922. Here, they initially delved into the fate of sugar within the animal body, exploring the impacts of insulin and epinephrine. They made groundbreaking discoveries, including the demonstration of glycolysis in tumours in vivo. Their research on carbohydrate metabolism evolved from whole animal studies to experiments on isolated tissues, and eventually to tissue extracts and isolated enzymes, including some in crystalline form. In a pivotal moment in 1936, they isolated glucose-1-phosphate, known as “Cori ester,” and linked its formation to the activity of phosphorylase, which plays a crucial role in the breakdown and synthesis of polysaccharides. This discovery paved the way for the enzymatic synthesis of glycogen and starch in vitro.
Their research extended into the realm of hormone action mechanisms, with several studies focusing on the pituitary gland. They observed significant changes in rats that have had their pituitary gland removed, including a marked decrease in glycogen and a drop in blood sugar levels, accompanied by an increased rate of glucose oxidation. Further investigations into the effects of hormones on hexokinase revealed that certain pituitary extracts could inhibit this enzyme both in vivo and in vitro, while insulin was found to counteract this inhibition.
Beyond their groundbreaking research, the Cori’s served as an endless source of inspiration to their peers in the vibrant hubs of biochemical research they led. Their contributions to The Journal of Biological Chemistry and numerous other scientific journals have left an indelible mark on the field, showcasing their innovative work and collaborative spirit throughout their careers.

Gertrude Belle Elion (1918-1999)
Gertrude provides us with another tale of the triumph over adversity. After graduating with a degree in biochemistry in 1937, she failed to obtain a graduate position because she was a woman. After roles in laboratories and teaching, she joined the Burroughs Wellcome Laboratories in 1944 and became the assistant to Dr George Hitchings. Over the following 40 years, the pair were successful in the development of a vast array of new drugs and treatments. Much of their success is attributed to their methods. At the time, normal practice is best described as trial and error. However, Hitchings and Elion studied and intimately understood the difference between normal and pathogenic biochemistry, allowing them to envision and create targeted treatments for, to name just a few, leukaemia, urinary-tract infection, gout, malaria and viral herpes.
Although she never achieved her doctorate, in 1967 Elion was promoted to Head of the Department of Experimental Therapy, where she remained until her retirement in 1983. But Elion didn’t let a silly old thing like retirement get in her way. She remained at the Burroughs Wellcome Laboratories as a Scientist Emeritus and Consultant, including overseeing the development of azidothymidine, the first drug used to treat AIDS. Elion also became a Research Professor of Medicine and Pharmacology at Duke University, working with medical students in the field of tumour biochemistry and pharmacology, while continuing to write and lecture. Gertrude B. Elion passed away in 1999, bringing an end to a happy and fruitful career as one of the most influential women in science.

Rosalind Franklin (1920-1958)
Rosalind Franklin, another well-known name and one synonymous with the discovery of the DNA double helix, was an exceptional scientist whose meticulous work in X-ray crystallography laid the groundwork for one of the 20th century’s most significant scientific discoveries. Franklin graduated from Cambridge University in 1941, where she initially delved into the study of coal, gases, and carbon compounds, significantly contributing to the understanding of the molecular structures of these materials. Her early research not only showcased her exceptional skills in physical chemistry but also set the stage for her pioneering work in biology.
In 1951, Franklin joined King’s College London, where she was tasked with improving the X-ray crystallography unit. It was here that Franklin embarked on her most famous work: the study of the structure of DNA. Using her expertise in X-ray diffraction techniques, she captured Photograph 51, a critical piece of evidence revealing the helical structure of DNA. This image was crucial in identifying the double helix structure, although her contributions were not fully acknowledged until after her death.
Franklin’s research extended beyond DNA to the study of viruses, making significant strides in understanding the polio virus and the tobacco mosaic virus. Her work in virology, much like her work on DNA, was pioneering, employing her crystallography skills to uncover the detailed structure of viral particles. This work provided valuable insights into how viruses replicate and infect cells, contributing to the broader field of virology and paving the way for future research in virus structure and function.
Despite facing considerable challenges as a woman in a predominantly male scientific community, Franklin’s contributions were profound. Her relentless pursuit of scientific truth, combined with her exceptional experimental skills, left a legacy in the fields of chemistry, virology, and genetics. Beyond her scientific achievements, Franklin is remembered as a trailblazer who paved the way for future generations of women in science, demonstrating the critical role of perseverance and dedication in the pursuit of knowledge.

Françoise Barré-Sinoussi (1947-)
When discussing women who changed science, it’s impossible not to mention Françoise Barré-Sinoussi. She was born in Paris in 1947 and attended university there. Her passion for science saw her skip class to work at the Pasteur Institute, participating in investigations of retroviruses that caused leukaemia in mice. Although, this didn’t seem to affect her exams scores, and she received her PhD in 1974.
Françoise Barré-Sinoussi is hailed as the woman who discovered the viral cause of the AIDS epidemic. In 1982, the Pasteur Institute was approached by a virologist from a hospital in Paris, seeking help in identifying the cause of a worrying new epidemic. In a mere 2 weeks, Barré-Sinoussi, and her colleagues at the Pasteur Institute, isolated and grew a retrovirus from a biopsied lymph node of a patient at risk of AIDS. The virus, later named HIV-1, was found to be the cause of the AIDS epidemic.
Barré-Sinoussi has been contributing to virology research ever since, including areas such as the function of the host’s innate immune defences in managing HIV/AIDS, the elements contributing to the transmission of HIV from mother to child, and traits enabling a select group of HIV-positive individuals to restrain HIV replication without the need for antiretroviral medications. In 1992, Barré-Sinoussi was appointed Head of the Biology of Retrovirus Unit, renamed the Regulation of Retroviral Infections Unit in 2005.
However, Barré-Sinoussi didn’t stop at the science. She became a prominent activist for public education about AIDS prevention and helped to establish centres for diagnosing and treating AIDS around the world. In 2006, Barré-Sinoussi was elected to the International AIDS Society (IAS) Governing Council and served as president of the IAS from 2012-2016.

Jennifer Douda and Emmanuelle Charpentier
Most people have heard of CRISPR-Cas9. But many aren’t aware that we have women to thank for this scientific innovation. In 2012, Jennifer Douda (left) and Emmanuelle Charpentier (right) discovered the ingenious CRISPR-Cas9 technology – a groundbreaking tool in genetic engineering, which holds the promise of revolutionising medicine and biology. By enabling precise editing of the DNA in the cells of living organisms, CRISPR-Cas9 could lead to cures for genetic disorders, enhance crop resistance to pests and diseases, and advance our understanding of complex genetic conditions. It offers the potential to correct genetic defects, combat infectious diseases, and even manipulate traits in plants and animals, paving the way for significant advancements in therapeutic treatments, agricultural productivity, and the study of genetics. This discovery saw both women share the Nobel Prize in Chemistry in 2020.


We’ve only looked at a subsection of science, but one where the importance of the innovations and discoveries is felt by people every day. The scientists we’ve discussed here are only a small sample of the inspirational women that have graced the STEM field. We hope that by elucidating some of their work, we can inspire more girls and women to pursue a career in STEM. Careers in this field are challenging and rewarding, perfect for those with a curious mind and those in whom discovery sparks delight.
World Hepatitis Day 2023
Introduction
World Hepatitis Day, observed on July 28th, serves as a crucial reminder of the ongoing battle against hepatitis (HBV), a viral infection that affects millions of people worldwide. In 2019, it was estimated that 296 million people were living with chronic hepatitis B, resulting in over 800,000 fatalities1. In this article, we will delve into the intricate mechanisms behind hepatitis, explore the viral species responsible for its occurrence, discuss methods for diagnosis, and shed light on treatment and management strategies.
Understanding Hepatitis
Hepatitis refers to the inflammation of the liver, often caused by viral infections. Among the primary hepatitis viruses are Hepatitis A, B, C, D, and E, each with distinct modes of transmission and characteristics2.
Mechanisms of Hepatitis Infection
Hepatitis A and E: Hepatitis A and E viruses are primarily transmitted via the faecal-oral route, often through contaminated food or water. Ingestion of these viruses leads to acute infection, and while self-limiting in most cases, they can cause significant morbidity and mortality in certain populations5,6.
Hepatitis B, C, and D: Hepatitis B, C, and D viruses are predominantly spread through blood and bodily fluids. Hepatitis B can also be transmitted from mother to child during childbirth which in endemic areas, HBV infection from mother to child transmission accounted for approximately half of chronic infections. These viruses can cause chronic infections, leading to long-term liver damage, cirrhosis, and an increased risk of hepatocellular carcinoma7,8.
Diagnosis of Hepatitis
Accurate and timely diagnosis of hepatitis is crucial for appropriate management. Diagnostic methods include:
Serology: Serological tests, such as enzyme immunoassays, are employed to detect specific viral antigens or antibodies in blood samples, aiding in the identification of different hepatitis viruses and determining the stage of infection9.
Nucleic Acid Testing: Highly sensitive molecular techniques like polymerase chain reaction (PCR) enable the detection and quantification of viral genetic material, aiding in the diagnosis and monitoring of chronic hepatitis10.
Treatment and Management of Hepatitis
The management of hepatitis depends on several factors, including the virus involved, the stage of infection, the presence of co-infections, and the individual patient’s health status. Treatment strategies encompass:
Antiviral Medications: For hepatitis B and C, antiviral drugs such as interferons and direct-acting antivirals have revolutionized the treatment landscape, offering higher cure rates and improved outcomes11,12.
Supportive Care: Hepatitis patients may require supportive care to alleviate symptoms, maintain proper nutrition, and manage complications. Vaccination against hepatitis A and B is highly recommended for prevention13.
Liver Transplantation: In cases of end-stage liver disease or hepatocellular carcinoma resulting from chronic hepatitis, liver transplantation may be considered a lifesaving option14.
Randox Hepatitis Solutions
Acusera
Acusera provides a range of positive and negative serology controls comprising various infectious diseases including Hepatitis. The table below details the suitable controls, and more information can be found on our website: Serology Quality Controls – Randox Laboratories

RIQAS
The RIQAS HIV/Hepatitis EQA programme is designed to monitor the performance of tests used to detect HIV/Hepatitis antibodies and specific antigens. All samples are conveniently supplied liquid ready-to-use and are suitable for qualitative methods of analysis.
Parameters:
- Anti-HIV-1
- Anti-HCV
- Anti-HTLV-II
- HBsAg
- Anti-HIV-2
- Anti-HBc
- Anti-HTLV-1&2 (combined)
- Anti-HIV-1&2 (combined)
- Anti-HTLV-I
- Anti-CMV
- Anti-HAV IgM
- Anti-HAV (Total)
- Anti-HBc (Total)
- Anti-HBe (Total)
- Anti-HBs (Total)
- P24
For more information, please visit our website at: HIV Hepatitis EQA | RIQAS (randox.com)
Qnostics
Monitoring for the presence of Blood Borne Virus (BBV) nucleic acid is an essential parameter in guiding clinical treatment and patient outcomes. The use of appropriate quality control measures is important in ensuring the appropriate daily performance of the molecular assay used in the laboratory independent of the technology.
Qnostics’ Blood Borne Virus Molecular Controls comprises a range of pathogens which are classically detected directly from the blood including those related to hepatitis. The table below lists the Qnostics products related to hepatitis testing. For more information visit our website: Qnostics | Molecular Infectious Disease Controls – Randox Laboratories

QCMD
QCMD is a world-leading External Quality Assessment (EQA) / Proficiency Testing (PT) scheme, dedicated to improving the quality of molecular diagnostic assays used in the detection of infectious diseases. With an extensive database of over 2000 participants in over 100 countries, QCMD is one of the largest providers of molecular EQA in the field of molecular diagnostics. QCMD programmes related to hepatitis testing are listed below:
- HBV Drug resistance Typing EQA programme.
- HCV Drug resistance Typing EQA programme.
- Hepatitis B Virus DNA EQA Programme
- Hepatitis B Virus Dried Blood Spot EQA Pilot Study
- Hepatitis B virus Genotype EQA Programme
- Hepatitis C Virus Dried Blood Spot EQA Pilot Study
- Hepatitis C Virus RNA EQA Programme
- Hepatitis C virus Genotype EQA Programme
- Hepatitis D Virus EQA Programme
- Hepatitis E virus RNA EQA Programme
For more information on any of these EQA programmes please visit: QCMD – Molecular EQA Scheme | Randox Quality Control
Conclusion
World Hepatitis Day serves as a reminder of the global impact of hepatitis and the urgent need to raise awareness, prevent transmission, and improve the diagnosis and management of this disease. By understanding the mechanisms, bacterial species involved, diagnostic techniques, and treatment approaches, we can work towards a future free from the burden of hepatitis. Let us unite in our efforts to combat this disease and strive for a healthier world.
If you’d like to find out more about hepatitis or the diagnosis and testing of hepatitis, please visit our website. If you’d like more information on how Randox can improve hepatitis testing in your laboratory, please reach out to marketing@randox.com.
References
- World Health Organization. World Health Statistics 2023. World Health Organization; 2023. https://www.who.int/publications/i/item/9789240074323
- World Health Organization. Hepatitis. https://www.who.int/news-room/fact-sheets/detail/hepatitis-a. Published 2017. Accessed June 9, 2023.
- Wan Z, Wang X. Bacterial Hepatitis. In: Encyclopedia of Medical Microbiology. Elsevier; 2020:110-117.
- Russo TA, McFadden DC. Bacterial and fungal infections in patients with cirrhosis. Clin Liver Dis. 2019;14(2):71-74.
- World Health Organization. Hepatitis E. https://www.who.int/news-room/fact-sheets/detail/hepatitis-e. Published 2018. Accessed June 9, 2023.
- Rakesh S, Pekamwar SS. Hepatitis A. In: StatPearls [Internet]. StatPearls Publishing; 2020.
- World Health Organization. Hepatitis B. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b. Published 2021. Accessed June 9, 2023.
- World Health Organization. Hepatitis D. https://www.who.int/news-room/fact-sheets/detail/hepatitis-d. Published 2021. Accessed June 9, 2023.
- Alfaresi MS, Elkoush AA, Khan AS. Serological diagnosis of viral hepatitis. J Clin Transl Hepatol. 2017;5(4):343-359.
- European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C. J Hepatol. 2017;66(1):153-194.
- European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370-398.
- Vermehren J, Sarrazin C. New HCV therapies on the horizon. Clin Microbiol Infect. 2011;17(2):122-134.
- World Health Organization. Hepatitis A. https://www.who.int/news-room/fact-sheets/detail/hepatitis-a. Published 2020. Accessed June 9, 2023.
- Kim WR, Terrault NA. Hepatocellular carcinoma and liver transplantation. Clin Liver Dis. 2018;22(2):381-394.











