Product Spotlight: HbA1c Quality Controls
Product Spotlight: HbA1c Quality Controls
Diabetes
Diabetes is a life-long condition which occurs when the glucose level in the blood is too high because it can’t enter the body’s cells to be used as fuel. There are two types of diabetes: type 1 and type 2. They are distinct conditions and must be treated and managed differently.
Type 1 Diabetes
Type one diabetes is an autoimmune condition in which the body attacks insulin-producing cells, this causes a lack of insulin, leading to an increased blood glucose level. Around 10% of people with diabetes has type 1.
Type 2 Diabetes
A mixture of genetic and environmental factors causes type 2 diabetes. The body doesn’t make enough insulin or the insulin it does create does not work correctly, leading to a glucose build up in the blood. It’s thought that up to 58% of type 2 diabetes can be prevented or delayed through healthy lifestyle choices.
HbA1c
HbA1c is the average blood glucose level for the past two to tthree months. A high HbA1c means there is too much sugar in the bloodstream. This means the patient is more likely to develop complications associated with diabetes, like problems with feet and eyes [1].
HbA1c in Diagnostics
In 2011, the WHO accepted the use of glycated haemoglobin (HbA1c) testing in the diagnosis of diabetes, Diabetes UK also supports this decsion [2].
Clinically Significant Levels
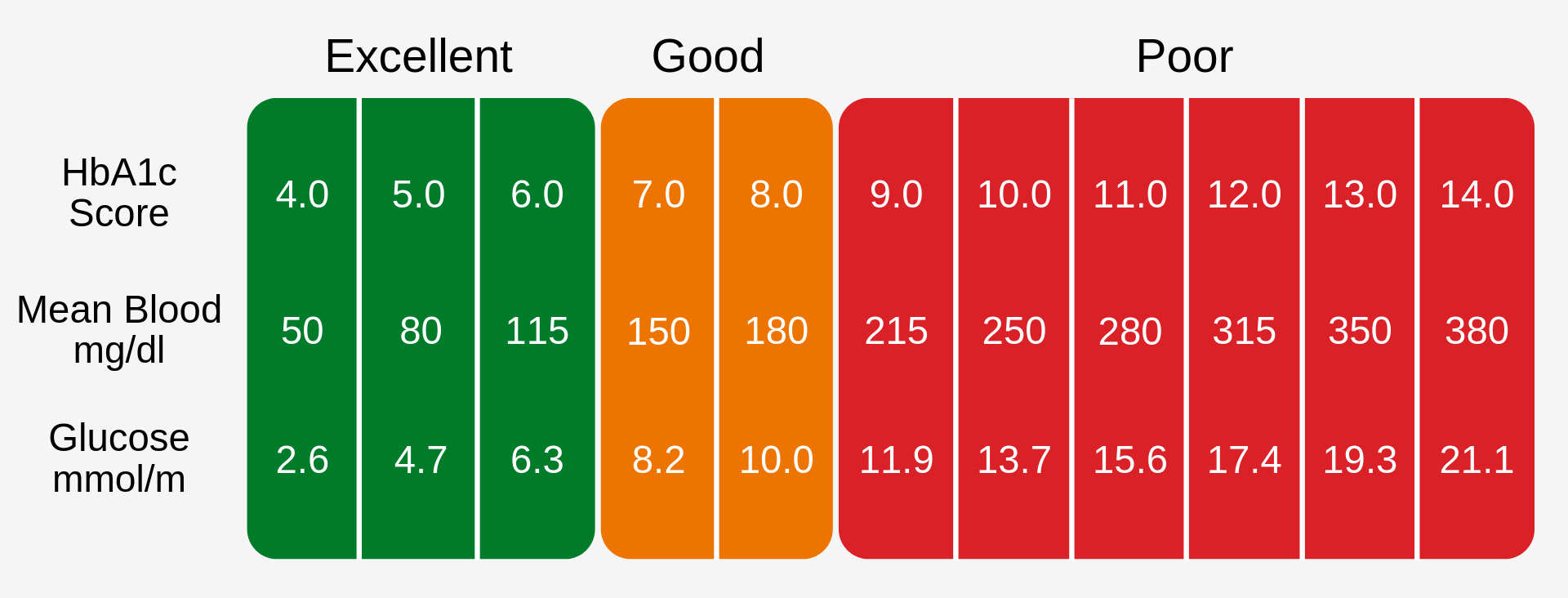
A HbA1c level of 6.5% is recomended as the cut off for diabetes diagnosis [3], this can be seen in Fig. 1.
Acusera HbA1c Controls
Acusera HbA1c Quality Control
The Randox Acusera HbA1c control is designed for use in the quality control of both HbA1c and Total Haemoglobin assays. Assayed instrument and method specific target values and ranges are provided for all major systems and methods including HPLC. A reconstituted stability of 4 weeks keeps waste to a minimum and helps to reduce costs.
Acusera Liquid HbA1c Quality Control
Conveniently supplied liquid ready-to-use the Liquid HbA1c control is ideally suited to both clinical laboratories and POCT helping to significantly reduce preparation time. With a stability of 30 days waste and costs are also kept to a minimum.
Flu Season
Molecular Respiratory Testing
Contact Us
Product Spotlight Home
Visit the Product spotlight Home to see past product spotlights
References
[1] “What is HbA1c?”, Diabetes UK, 2018. [Online] Availabel: https://www.diabetes.org.uk/guide-to-diabetes/managing-you-diabetes/hba1c.
[2] Diabetes UK, “Diagnostics criteria for deabetes”, Diabetes UK, 2018. [Online]. Available: https://www.diabetes.org.uk/professionals/position-statements-reports/diagnosis-ongoing-management-monitoring/new_diagnostic_criteria_for_diabetes.
[3] WHO, “Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus”, World Health Organisation, 2011.
Product Spotlight: Respiratory Controls – Molecular Infectious Disease Testing



Product Spotlight
Molecular Controls for Respiratory Infection Testing
Flu is a contagious respiratory illness cause by influenza viruses that infect the throat, nose, and sometimes lungs. It can cause illness and sometimes death. Getting vaccinated is the best way to prevent catching flu [1].
There are four types of seasonal flu, A, B, C, and D. Types A and B cause seasonal epidemics of disease. Illnesses range from severe to mild and can even result in death in high risk groups. High risk groups include, pregnant women, children under 5 years of age, the elderly, and people with chronic or immunosuppressive medical conditions [2].
Flu season begins as early as October, reaches its peak in February, and ends in March. In the southern hemisphere, flu season falls between June and September. Wherever it’s cold, it’s flu season.
Diagnosing Flu
A test to detect Influenza viruses can be used to determine whether a patient has the flu. A swab is taken from either the nose or back of the throat and sent for testing. Molecular assays can be used to detect genetic material of the virus [3]. Molecular methods play an important role in the diagnosis and surveillance of influenza viruses. Molecular diagnostics allow timely and accurate detection of influenza and are already implemented in many laboratories. The combination of automated purification of nucleic acids with real-time PCR should enable even more rapid identification of viral pathogens such as influenza viruses in clinical material [4].
The Qnostics range of complete molecular controls for infectious disease testing can be used in the daily monitoring of assay performance, linearity assessment, assay evaluation, validation/verification of new assays and staff training. As whole pathogen controls, the range is designed to mimic the performance of patient samples and can be used to effectively monitor the entire testing process including extraction, amplification and detection.
Qnostics offers a range of solutions for molecular respiratory testing:
Q Controls
Independently manufactured, these positive and externally run controls are designed to be treated as a patient sample within an assay run. Helping to support a laboratory’s accreditation requirements in line with ISO 15189:2012, Q Controls are supplied in an unassayed, liquid frozen format delivering accurate and reliable test results.
Analytical Q Panels
Each Analytical Q Panel consists of five or more individual samples including a negative and is designed to cover the dynamic range of individual infectious disease assays, in a linear progression. Analytical Q Panels are intended for use in the validation and verification of new assays with the main purpose of helping to ensure assays are linear throughout the dynamic range. In addition, Analytical Q Panels will support a laboratory’s accreditation requirements, in line with ISO 15189:2012.
Evaluation Panels
Evaluation Panels may be used to evaluate assay characteristics, confirm performance claims and ultimately ensure the assay is fit for purpose. Evaluation Panels may also be used in the validation of clinical assays and the development of diagnostic tests.
Flu Season
Molecular Respiratory Testing
Contact Us
Product Spotlight Home
Visit the Product spotlight Home to see past product spotlights
References
[1] “Key Facts About Influenza (Flu) | Seasonal Influenza (Flu) | CDC”, Cdc.gov, 2018. [Online]. Available: https://www.cdc.gov/flu/keyfacts.htm. [Accessed: 25- Sep- 2018].
[2] “Influenza (Seasonal)”, World Health Organization, 2018. [Online]. Available: http://www.who.int/en/news-room/fact-sheets/detail/influenza-(seasonal). [Accessed: 27- Sep- 2018].
[3] “Diagnosing Flu | Seasonal Influenza (Flu) | CDC”, Cdc.gov, 2018. [Online]. Available: https://www.cdc.gov/flu/about/qa/testing.htm. [Accessed: 25- Sep- 2018].
[4] J. Ellis and M. Zambon, “Molecular diagnosis of influenza”, Reviews in Medical Virology, vol. 12, no. 6, pp. 375-389, 2002.
Product Spotlight: Acusera Cardiac Quality Control


For this month’s product spotlight, we have chosen our whole range of cardiac quality control solutions. Randox offers options for both Internal Quality Control (IQC) and External Quality Assessment (EQA).
29th September is World Heart Day. World Heart Day is a global campaign during which individuals, families, communities and governments around the world participate in activities to take charge of their heart health and that of others.
Cardiovascular disease (CVD) is a term to describe conditions that affect the heart and blood vessels. It is normally associated with a build up of fatty deposits in the arteries and an increased risk of blood clots. CVD is the leading casues of death globally, representing around 30% of all deaths. A healthy lifestyle can help prevent CVD; however, increasingly busy lifestyles mean that it is difficult to avoid CVD. This means it is vitally important that individuals are tested for CVD and to ensure that diagnoses are acurate.


The accurate diagnosis of a potentially life threatening cardiac event is essential in order to avoid misdiagnosis and/or incorrect treatment. The Acusera Cardiac Quality Controls have been designed to cover a wide range of cardiac markers at clinical decision levels eliminating the need for additional low level controls at extra expense. Available in a choice of both liquid ready-to-use and lyophilised formats, they are ideal for use at the point-of-care and in the laboratory. Manufactured from 100% human serum a matrix similar to that of the patient is guaranteed.
Liquid Controls
Liquid Cardiac Control
Liquid, Assayed, 100% Human serum
8 AnalytesBNP Control
Liquid, Assayed, 100% Human serum
1 AnalyteLyophilised Controls
Cardiac Control
Lyophilised, Assayed, 100% Human serum
7 AnalytesCK-MB Control
Lyophilised, Assayed, 100% Human serum
2 AnalytesH-FABP Control
Lyophilised, Assayed, 100% Human serum
1 AnalytehsTnT Control
Lyophilised, Assayed, 100% Human serum
1 Analyte
The RIQAS Cardiac EQA programme is designed to monitor the performance of up to 7 clinically significant cardiac markers. Two flexible reporting options are available to suit the needs of all laboratory sizes.
Accredited to ISO/IEC 17043
• 100% human serum
• Bi-weekly reporting
• Submit results and view reports online via RIQAS.Net
• Register up to five instruments at no extra cost
Parameters
CK, Total | CK-MB activity units | CK-MB mass units | Homocysteine | Myoglobin | Troponin I | Troponin T
Cardiac Controls
Cardiac EQA
Contact Us
Product Spotlight Home
Visit the Product spotlight Home to see past product spotlights
Product Spotlight: Acusera Immunoassay Premium Plus Control


The Randox Acusera Immunoassay Premium Plus quality control covers an impressive 54 analytes with assayed instrument specific target values and ranges provided for up to 51 analytes. The unique combination of routine tumour markers, therapeutic drugs and Vitamin D allows laboratories to dramatically reduce the number of controls required while ultimately keeping costs to a minimum. Three levels of control are available with analytes present at clinically significant levels.
Features & Benefits
- Lyophilised for enhanced stability
- 100% human serum
- Assayed, instrument specific target values provided for up to 51 parameters
- Ferritin and Vitamin B12 levels suitable for anaemia monitoring
- Ultra low TSH levels in the level 1 control
- Stable to expiry date at 2°C – 8°C
- Reconstituted stability of 7 days at 2°C – 8°C or 4 weeks at -20°C
- Contains routinely run tumour markers: AFP/CA15-3/CA19-9/CA-125/CEA/PSA/Free-PSA
Flexible options are available with a low, normal and high level, as well as a combined tri-level.
Description |
Size |
Analytes |
Cat No. |
| Immunoassay Premium Plus Level 1 | 12 x 5 ml | 54 | IA3109 |
| Immunoassay Premium Plus Level 2 | 12 x 5 ml | 54 | IA3110 |
| Immunoassay Premium Plus Level 3 | 12 x 5 ml | 54 | IA3111 |
| Immunoassay Premium Plus Tri-Level | 4 x 3 x 5 ml | 54 | IA3112 |
The clinical significance of some selected analytes.
Androstenedione
Androstenedione is an androgen, which are hormones responsible for the induction of sexual differentiation and produce secondary male physical characteristics such as a deep voice and facial hair. Another example is testosterone. They are also present in females as precursors to female hormones (such as estrogen).
This test measures the amount of androstenedione in the blood. Because AD has its origins in the adrenal glands, it is useful as a marker of adrenal gland function, of the function of the ovaries or testicles, and androgen production.
An androstenedione level may be used to [1]:
• Evaluate adrenal gland function and distinguish between androgen-secreting conditions that are caused by the adrenal glands from those that originate in the ovaries or testicles, if results of DHEAS and testosterone testing are abnormal • Help diagnose tumours in the outer layer (cortex) of the adrenal gland or tumours outside of the adrenal gland that secrete ACTH (ectopic) and separate these conditions from ovarian or testicular tumours and cancers • Diagnose congenital adrenal hyperplasia (CAH) and monitor CAH treatment, in addition to tests for testosterone and 17-hydroxyprogesterone • Help diagnose polycystic ovarian syndrome (PCOS) and help rule out other causes of infertility, no monthly menstrual periods (amenorrhea), and excess body and facial hair (hirsutism) in women who have abnormal results on tests for DHEAS, testosterone, and other hormones such as FSH, LH, prolactin, and estrogen • Help determine the cause of delayed puberty and investigate suspected ovarian or testicular failure • Investigate and diagnose the cause of male physical characteristics (virilisation) in young girls and early (precocious) puberty in young boys Digoxin
Digoxin is a therapeutic drug used to treat abnormal heart rhythms and failure. Heart failure causes the heart to become less effective at circulating blood, resulting in blood backing up in the hands, legs, liver, and lungs, causing swelling.
This test measures the level of digoxin in the blood. Digoxin is prescribed to patients to alleviate symptoms of heart failure by strengthening contractions, helping the heart pump blood more effectively. Digoxin can also be used to treat arrhythmias. It cannot be used to cure heart failure or arrhythmias but can help to manage the symptoms along with other medication, exercise and diet.
Digoxin levels are monitored due to the drug’s narrow safety range. If the level is too high, toxicity may occur, if too low, symptoms may recur.
• Monitor the concentration of the drug in the patient's blood • Determine if a patient's symptoms are due to insufficient levels of Digoxin or due to digoxin toxicity • Increase the strength and efficiency of heart contractions, helping control the rate and rhythm of the heart • Slows electrical conduction between the atria and the ventricles, which is useful in treating abnormally rapid atrial rhythms that can cause heart attacks Luteinizing Hormone (LH)
Luteinizing Hormone (LH) is associated with the reproductive cycle and the release of an egg from the ovary in women and testosterone production in men. LH production is a complex system controlled by the hypothalamus in the brain, hormones produced in the testes and ovaries, and the pituitary gland.
This test measures the amount of luteinizing hormone in the blood or urine. It is used alongside other tests such as FSH, estradiol, progesterone, and testosterone to investigate reproductive irregularities.
A luteinizing hormone level may be used [4]:
In both men and women:
• In the workup of infertility • To aid in the diagnosis of pituitary disorders that can affect LH production • To help diagnose conditions associated with dysfunction of the ovaries or testicles In women:
• In the investigation of menstrual irregularities • To detect a surge in LH levels during the menstrual cycle, helping determine when a woman is likely to be the most fertile In children:
• To diagnose delayed and precocious (early) puberty. Irregular timing of puberty may be an indication of a more serious problem involving the hypothalamus, the pituitary gland, the ovaries or testicles, or other systems Insulin
Insulin is a hormone produced and stored in the beta cells of the pancreas. It is secreted as a response to an elevated glucose level following a meal and is vital in the transportation and storage of glucose, the body’s primary source of energy. Insulin regulates blood glucose by helping transport it from blood to cells.
This test measures the amount of insulin in the blood. After a meal, carbohydrates are broken down into glucose, this is then absorbed into the blood causing the blood glucose level to rise, this then falls as it moves into cells. If an individual cannot produce enough insulin, or if they are insulin resistant (cells are resistant to insulin’s effects), glucose does not reach the body’s cells and they starve. The blood glucose level will rise to an unhealthy level, which can cause various complications, including Diabetes.
An insulin level may be used to [5]:
• Diagnose an insulinoma, verify that removal of the tumor has been successful, and/or to monitor for recurrence • Diagnose the cause of hypoglycemia in an individual with signs and symptoms • Identify insulin resistance • Monitor the amount of insulin produced by the beta cells in the pancreas (endogenous); in this case, a C-peptide test may also be done. Insulin and C-peptide are produced by the body at the same rate as part of the conversion of proinsulin to insulin in the pancreas • Determine when a type 2 diabetic might need to start taking insulin to supplement oral medications • Determine and monitor the success of an islet cell transplant intended to restore the ability to make insulin, by measuring the insulin-producing capacity of the transplant What test results mean [5]:
DisorderFasting Insulin LevelFasting Glucose LevelInsulin resistance High Normal or somewhat elevated Not enough insulin produced in beta cells (diabetes, pancreatitis) Low High Hypoglycemia due to excess insulin (insulinomas, cushing syndrome) Normal or High Low
Immunoassay Controls
Immunoassay EQA
Contact Us
References
[1] “Androstenedione”, Labtestsonline.org, 2017. [Online]. Available: https://labtestsonline.org/tests/androstenedione. [Accessed: 07- Aug- 2018].
[2] “Digoxin”, Labtestsonline.org, 2017. [Online]. Available: https://labtestsonline.org/tests/digoxin. [Accessed: 08- Aug- 2018].
[3] O. Ogbru and J. Marks, “digoxin, Lanoxin: Drug Facts, Side Effects and Dosing”, MedicineNet. [Online]. Available: https://www.medicinenet.com/digoxin/article.htm#which_drugs_or_supplements_interact_with_digoxin?. [Accessed: 08- Aug- 2018].
[4] “Luteinizing Hormone (LH)”, Labtestsonline.org, 2017. [Online]. Available: https://labtestsonline.org/tests/luteinizing-hormone-lh. [Accessed: 08- Aug- 2018].
[5] “Insulin”, Labtestsonline.org, 2017. [Online]. Available: https://labtestsonline.org/tests/insulin. [Accessed: 08- Aug- 2018].
Product Spotlight Home
Visit the Product spotlight Home to see past product spotlights
Product Spotlight: Acusera Immunoassay Speciality 2 Control


The Randox Acusera Immunoassay Speciality II quality control is designed to complement our Immunoassay Premium and Premium Plus controls. Assayed, instrument specific target values and ranges are provided for 4 complex immunoassay parameters.
Features & Benefits
- Lyophilised for enhanced stability
- 100% human serum
- Assayed target values provided for 4 parameters
- Stable to expiry date at 2°C – 8°C
- Reconstituted stability of up to 5 days at 2°C – 8°C and 4 weeks at -20°C
Procalcitonin
Procalcitonin (PCT), a protein that consists of 116 amino acids, is the peptide precursor of calcitonin, a hormone that is synthesized by the parafollicular C cells of the thyroid and involved in calcium homeostasis.
Procalcitonin is also produced by the neuroendocrine cells of the lung and intestine and is released as an acute-phase reactant in response to inflammatory stimuli, especially those of bacterial origin. This raised procalcitonin level during inflammation is associated with bacterial endotoxin and inflammatory cytokines.
Procalcitonin (PCT) versus C-reactive protein (CRP)
CRP is the most common laboratory marker used in the clinical setting to evaluate systemic inflammatory response to an infectious agent.
Procalcitonin is a more useful diagnostic inflammation parameter than CRP in patients with pediatric neutropenic fever, both in estimating the severity of infection and the duration and origin of the fever. Procalcitonin is also a more reliable parameter in the diagnosis of bacterial sepsis, allowing better differentiation among sepsis-related fatalities.[2]
Calcitonin
A calcitonin assay is helpful in identifying patients with nodular thyroid disease. It is often performed in the hope of identifying early MTC. Successful treatment of MTC depends on early detection; late detection confers a poor prognosis. In addition, calcitonin levels are reported to be increased in other malignancies, such as carcinoid tumors, lung carcinoma, melanoma, pancreatic and breast carcinoma, and pheochromocytoma.
Calcitonin is produced and released by parafollicular cells of the thyroid. Calcitonin is derived from larger precursors. Precalcitonin is cleaved to procalcitonin, which is further cleaved to immature calcitonin and then to mature calcitonin, a monomer of a 3.5-kd peptide composed of 32 amino acids, which is the only biologically active form.
Calcitonin’s precise physiologic role in humans remains to fully understood. It is known to act on the bones, kidneys, and gastrointestinal tract, binding directly to osteoclasts, thereby directly inhibiting osteoclastic bone resorption. Although this inhibition may be important in short-term control of calcium loads, it is short-lived and likely plays an insignificant role in overall calcium homeostasis. Calcitonin also inhibits the action of parathyroid hormone and vitamin D. [1]
Gastrin
Gastrin testing is employed in the diagnosis of gastrinoma, either with or without Zollinger-Ellison syndrome, and in the investigation of pernicious anemia and achlorhydria.
In patients who have undergone antrectomy with vagotomy, gastrin levels are reduced. In contrast, gastrin levels are increased in Zollinger-Ellison syndrome.[3]
Renin
Renin, also known as Angiotensinogenase, is an enzyme involved in the renin–angiotensin aldosterone system (RAAS), which regulates the body’s water balance and blood pressure level. The system regulates the extracellular volume in the blood plasma, lymph and interstitial fluid, as well as controlling constriction of the arteries and blood vessels.[5] It is secreted by the kidney from specialized cells called granular cells and has a fundamental role in hypertension development. Over activation of this system contributes to hypertension and associated end-organ damage.[4]
The secretion of renin is stimulated by the following three factors:
-
- When a fall in arterial blood pressure is detected by pressure sensitive receptors (baroreceptors) in the arterial vessels.
- When a decrease in sodium chloride (salt) is detected in the kidney by the macula densa in the juxtaglomerular apparatus.
- When sympathetic nervous system activity is detected through beta1 adrenergic receptors.[4]
-
Immunoassay Controls
Immunoassay Spec II EQA
Contact Us
References
[1]A. Sofronescu and E. Staros, “Calcitonin”, Emedicine.medscape.com, 2015. [Online]. Available: https://emedicine.medscape.com/article/2087580-overview#a4. [Accessed: 16- Jul- 2018].
[2]J. Lin, S. Yap and E. Staros, “Procalcitonin”, Emedicine.medscape.com, 2015. [Online]. Available: https://emedicine.medscape.com/article/2096589-overview#a4. [Accessed: 16- Jul- 2018].
[3]B. Devkota and E. Staros, “Gastrin”, Emedicine.medscape.com, 2014. [Online]. Available: https://emedicine.medscape.com/article/2089092-overview#a4. [Accessed: 16- Jul- 2018].
[4]T. Meštrović, “Renin Clinical Applications”, News-Medical.net, 2015. [Online]. Available: https://www.news-medical.net/health/Renin-Clinical-Applications.aspx. [Accessed: 16- Jul- 2018].
[5]D. Mandal, “Renin (Angiotensinogenase)”, News-Medical.net, 2014. [Online]. Available: https://www.news-medical.net/health/Renin-Angiotensinogenase.aspx. [Accessed: 16- Jul- 2018].
Product Spotlight Home
Visit the Product spotlight Home to see past product spotlights