Acusera 24.7 – Interlaboratory Data Management Reporting Software
Acusera 24.7 – Interlaboratory Data Management Reporting Software
Acusera 24•7 is a live, cloud based interlaboratory data management and peer group reporting software, intended to assist in the management of daily QC activities.
Designed to complement our range of Acusera true third party controls.
Key Benefits of Acusera 24•7
Peer Group Statistics
Updated live in real-time, labs can compare statistics to their peers and identify any unique or widespread issues to help reduce time and money spent on troubleshooting.
Fully Interactive Charts
Ability to compare performance, assess trends and monitor performance with automatically generated Levey-Jennings, Histograms and Performance Summary Charts.
Highly Flexible
Labs can configure their own performance limits, multi-rules, consensus groups and target values for each instrument or lot of QC.
Comprehensive Reports
Comprehensive, easy-to-read and customisable reports have been designed to speed up the review process and include Statistical Metrics, Uncertainty of Measurement, Exception & more.
24/7 Access
Gain access to this cloud-based software at anytime, anywhere. Removing the need installation, software maintenance and frequent back-ups.
Advanced Statistical Analysis
Automatically calculating advanced statistics including %Bias, Total Error and Sigma Metrics helps enhance performance assessment, improve QC strategy, and meet regulatory requirements.
Unique Dashboard Interface
Reduce time spent analysing data as instant identification of any QC failures over the past 7 days and alerts of product expiry are highlighted in the unique dashboard design.
Automated Data Import
Upload QC data directly to Acusera 24•7 via LIMS and middleware. This automated function eliminates the need for manual data entry and helps save money whilst increasing efficiency.
Technical Support
Expert technical support is available for users. Laboratories can access this service for troubleshooting purposes and therefore eliminates the need for on-site assistance.
Features of Acusera 24•7
The fast, intelligent user interface on 24•7 enables rapid review of QC data and QC failures, reducing the time spent analysing and identifying errors.
The new and improved home screen allows users to carry out tasks such as: Creation of new tests/panels | Filtering results by analyte/ instrument/ lot number | Entering single or several QC test(s) without having to navigate away to multiple areas within the application.
Specifically designed to speed up the review process, our comprehensive range of easy-to read reports include: Data Review | Exception Report | Statistical Analysis Report | Statistical Metrics Report
Customise reports to show data for a specific date range aswell as a particular test or instrument.
Instant access to interactive Levey-Jennings, Histogram and Performance Summary Charts as well as the ability to add events and multiple data sets to a single chart allows for swift performance monitoring and superior identification of trends across multiple instruments.
One location for all of your statistical analysis needs.
Resources
How To Guide: Logging in
How To Guide: Adding Events
How To Guide: Adding QC Panel
How To Guide: Adding QC Tests
How To Guide: Chart Generation and Overview
How To Guide: Data Entry Options
How To Guide: Exception Report
How To Guide: Home Page
How To Guide: Manual Data Entry
How To Guide: Navigating Side Bars
How To Guide: Peer Group Statistics
How To Guide: Result History
How To Guide: Statistical Analysis Report
How To Guide: Statistical Metrics Report
How To Guide: Uncertainty of Measurement
We Are Here To help
To learn more about how 24•7 works as an interlaboratory data management system/ peer group reporting software. Or, to see the software in action, click below or visit our video demonstrations now.

Hello, are you interested in learning more about Acusera 24.7 software?
See our options below:
What The Users Say:
“We have been using 24.7 as our IQC management solution for some time now after changing over from a different provider. To begin with the change was met with resistance, and I have to admit that I was one of those people. Change always is challenging, and it took time to adapt to a newer and different system.
However, over time, and with Randox’s assistance, 24.7 has become part and parcel of everyday IQC monitoring and management. From the ease of use of configuring new instruments, assays, panels and lot numbers to the plethora of data that can be extracted to aid in optimal IQC management, 24.7 offers the flexibility to adapt to any user organisation.”
Nuffield Health – United Kingdom
Continue Reading
Acusera 24•7 FAQs
Acusera 24•7 Login
24•7 Brochure
Contact Us
Randox International Quality Assessment Scheme (RIQAS)
RIQAS is the world’s largest External Quality Assessment scheme with more than 75,000 laboratory participants spanning over 138 countries.
Key Benefits of RIQAS
Cost Effective
Consolidate EQA analysis, reduce workload and costs with our comprehensive, multi-parameter programmes.
Flexible Programme Options
Flexible programme options are available to suit all laboratory budgets. The option to register up to five instruments per programme (volume permitting) at no extra cost for comparative performance assessment
Accredited
RIQAS is accredited to ISO/IEC 17043:2010 “Conformity Assessment – General Requirements for Proficiency Testing” which is accepted by national and international accreditation bodies. Please see accreditation schedule for additional information.
Expansive Peer Group Database
The availability of large peer groups ensures robust data is available for a wide range of instruments and methods.
Rapid, User-Friendly Reports
Our rapid, user-friendly reports allow at-a-glance performance assessment. Complimentary multi-instrument, inter-laboratory and end-of-cycle reports are also available.
Commutable Samples
A commutable sample matrix increases confidence that performance mimics that of patient samples.
Early Identification of Test System Errors
Frequent reporting allows early identification of test system errors, while our 72 hour report turnaround enables corrective action to be taken with minimum disruption to the lab.
Convenient Data Upload System
RIQAS.net is a convenient, web-based data entry system which allows participants to return results and view reports online.
High Quality Samples
EQA samples are manufactured to the highest quality standard and span clinically relevant levels.
RIQAS EQA Programmes
Choice & flexibility are guaranteed with our 36 programme portfolio.
Browse the programmes below
RIQAS EQA Reports
User-friendly, one-page per parameter reports allow for at-a-glance performance assessment.
Browse the reports below.
What Does RIQAS Provide?

RIQAS Calendar 2024
What the Participants say:
“EcoScense LLC joined RIQAS in 2015 and currently run the General Clinical Chemistry, Haematology and Microbiology Programmes. We chose RIQAS as it is the best quality and has the most accessible programmes with weekly and monthly reporting available. We are very satisfied with the service and support we receive from the Randox team.”
EcoSenseLLC – Aremnia
“Alfa Diagnostica have been participating in RIQAS for 6 years. Over this time, the number of programmes has increased from 4 to 12, including Haematology, Immunoassay, Coagulation and Urinalysis. We chose RIQAS because of its quality, well-thought-out programmes, convenient scheduling and the large number of participants. RIQAS has enhanced the quality of our services by providing a flexible, convenient and objective instrument for self-evaluation. It has served as proof of reliable results for any interested third party.”
Alfa Diagnostica SRL – Republic of Moldova
Continue Reading
RIQAS Brochure
RIQAS Parameters
RIQAS.net
RIQAS Calendar
Contact Us
RIQAS Past Panels
RIQAS (Randox International Quality Assessment Scheme) is the largest global EQA scheme with over 50,000 participants in more than 139 countries. Our range currently comprises 36 programmes and the majority of clinical testing.
What are RIQAS Past Panels?
Available Panels
• Ammonia/ Ethanol
• Anti-Müllerian Hormone (AMH)
• Anti-TSH Receptor
• Blood Gas
• BNP
• Cardiac
• Cardiac Plus
• Cerebrospinal Fluid (CSF)
• Coagulation
• CO-Oximetry
• CYFRA 21-1
• Cytokines
• ESR
• General Clinical Chemistry
• Glycated Haemoglobin (HbA1c)
• Haematology
• Human Urine
• Immunoassay
• Immunoassay Speciality 1
• Immunoassay Speciality 2
• Immunosuppressant
• Lipid
• Maternal Screening
• Microbiology (Bacterial Identification)
• Neonatal Bilirubin
• Serology (Anti-SARS-CoV-2)
• Serology (EBV)
• Serology (HIV/ Hepatitis)
• Serology (Syphilis)
• Serology (ToRCH)
• Serum Indices
• Specific Proteins
• Sweat Testing
• Therapeutic Drugs
• Urinalysis
• Urine Toxicology
*Product availability is dependent on RIQAS stock surplus levels.
Benefits of RIQAS Past Panels
All RIQAS Past Panels are provided with an Excel document containing supporting data for the instrument and method of interest.
RIQAS Past Panel samples span multiple levels, making them ideal for ensuring accurate instrument performance across the measuring range.
RIQAS Past Panels allow laboratories to receive comprehensive external QC reports even after program close.
RIQAS Serology Report
The Serology report is available within ten days of the final submission date. The results for each parameter are displayed on a single page report. There are two types of Serology report – one for qualitative reporting and one for quantitative reporting. Each of these report ensure quick and easy performance assessment.
RIQAS EQA Reports
The Qualitative report comprises three subsections including a text section, Histogram and method summary. The text section conveniently displays your laboratory’s result to the correct result for your method. The Histogram visually displays your laboratory’s result in relation to all of the results submitted for your method group and the all method group. Finally, the method summary provides a breakdown of the results for all methods registered with RIQAS.
The Quantitative report comprises four subsections including a text section, Histogram, Levey-Jennings chart and method summary. The text section conveniently displays your laboratory’s result to the mean for comparison, the histogram visually displays your laboratory’s result to the mean for comparison, the Levey-Jennings chart displays the SD for the last 20 samples ensuring instant identification of performance over time and finally the method summary provides a breakdown of the results submitted for all methods registered with RIQAS.
RIQAS Urinalysis Report
RIQAS Urinalysis reports have been designed specifically for participants of the RIQAS Urinalysis programme (RQ9138). Report features include;
- Scoring system
- Ability to rate performance and visualise historical performance data
- Visual representation of performance
- Peer group comparison levels
To find out more on the range of features available with the report, see the table below.
RIQAS EQA Reports
The Urinalysis Report scoring system, scores participants based on a spread of results over each category and how far a participant is away from the consensus, which is referred to as the Target Category.
The score calculated is based on how close to the target category a participant’s result falls. A score of 0 refers to a result which falls within the target category and therefore, the comment “acceptable” will be given. A laboratory’s performance is described as being acceptable or unacceptable based on this calculated score.
A laboratory must achieve a score of between -6 and +6 for their performance to be deemed acceptable. Scores can fall between the values of -10 and +8.
- If a participant’s result matches the target category then they will receive the score of 0 and the comment “acceptable” given.
- Where a participant returns a negative result and the target category is positive (category 9), the participant score will be -10. The comment “unacceptable” will be given.
- Where a participant returns a positive result (category 9) and the target category is negative, the participant score will be +8 and the comment “unacceptable” given.
Current Sample
The score achieved for the current reported sample together with the associated comment of acceptable, unacceptable or borderline will be listed in the comments box located towards the top of the results page for each registered parameter. The percentage (%) of results from the selected peer group which fall within the target category are also stated.
Potential Historical Information
The number of correct scores (i.e. scores of 0) that have been submitted in the last “n” samples, up to a maximum of 6 previous samples. The number of acceptable assessments that have been submitted in the last “n” samples, up to a maximum of 6 previous samples.
Charting of Current & Historical Information
Results of scoring for each sample are displayed on a Levey-Jennings style chart with “0” score in the middle and scoring categories positioned on either side for 2, 4, 6, 8 and 10. The chart is shaded into bands depending on the scores.
Acceptable scores (0-6) have no shading, Borderline scores (6-8) have light red shading and unacceptable scores (8-10) are shaded a darker red.
Scores for each of the last 12 samples are plotted against the appropriate sample number and the chart also indicates whether the participant’s reported result category is higher or lower than the target category.
The target categories are stated along the top of the chart and if there are no target categories due to a lack of numbers then an “X” is plotted to show that a result has been submitted but could not be scored.
Reporting – Summary Page
The summary page at the end of the report lists all of the parameters that a participant has registered for.
For each parameter the following information is stated:
- Target Category, which is dependent on the unit registered
- The result which has returned by the participant
- The score achieved for the current sample
- The comment of Acceptable / Unacceptable / Borderline
- Where the comment is “Unacceptable” it is highlighted in bold italics and underlined
- The number of “Acceptable” assessments that have been achieved over the previous 6 samples
RIQAS Urine Toxicology Report
A dedicated report is provided for the Urine Toxicology programme. The report is divided into two subsections; the screening (qualitative) and the quantitative section, allowing for visual performance assessment at-a-glance.
RIQAS EQA Reports
Your laboratory’s result is displayed along with the correct response. A visual chart is also provided displaying performance of the last 20 samples. A breakdown of the screening results submitted by other laboratories in your method group and all method groups for the current sample is also provided at a variety of cut-offs. A further breakdown is provided for various methods at your specific cut-off.
The Quantitative section of the Urine Toxicology report is further broken down into a text section comparing your laboratory’s performance to the mean for comparison, a Histogram chart indicating your laboratory’s performance in relation to the method group and the all method group and a multi-method section highlighting the performance of other methods.
RIQAS Instrument Group Report
All RIQAS participants are entitled to register up to five separate instruments per programme at no extra cost.
Individual reports for each instrument are supplied as normal in addition to a complimentary instrument group report allowing the performance of each instrument to be uniquely compared and assessed easily.
RIQAS EQA Reports
The multi-instrument group report displays the %Deviation for the last 10 samples along with the RM%Dev across all registered laboratory instruments. The %Dev for each instrument is plotted on a user-friendly, colour coded chart for instant identification of poor performing instruments.
As an ISO requirement, the Multi-Instrument Report is an extremely useful and beneficial tool for laboratories – “Laboratories with two or more analysers for examinations, should have a defined mechanism for comparison of results across analysers” – ISO 15189:2012.
RIQAS Inter-Laboratory Group Report
A unique group reporting facility is available allowing laboratory groups or chains to effectively monitor the performance of satellite sites.
All laboratories within a group will receive their RIQAS sample packs, analyse the samples, return results to RIQAS and receive their individual RIQAS reports as normal. In addition to this a separate group report will be sent to the group manager or supervisor allowing relative performance of all laboratories within the group to be assessed.
RIQAS EQA Reports
RIQAS End-of-Cycle Report
RIQAS End-of-Cycle reports are sent to all participants who receive the standard report. Therefore, participants who are enrolled in RIQAS Urinalysis, RIQAS Urine Toxicology and RIQAS Serology programmes will not receive an End-of-Cycle report.
The RIQAS End-of-Cycle report provides laboratories with a complete summary of their External Quality Assessment performance. Performance is also compared to that of the previous cycle providing an indication of progress and improvement over time. Data is presented in both written and graphical format allowing a visual assessment of overall performance.
The RIQAS End-of-Cycle report is split into 3 sub-sections. These sub-sections are designed to allow performance assessment at a glance.
RIQAS EQA Reports
RIQAS End-of-Cycle Report Features
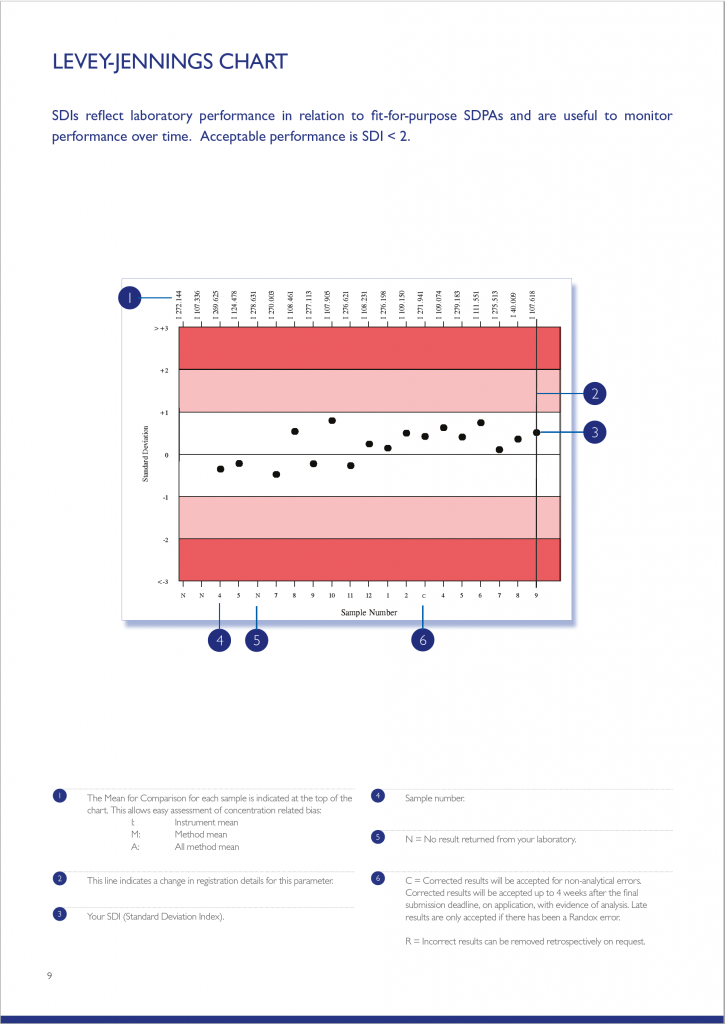
The Parameter Cycle Summary section provides an overview of the results returned for each sample in the cycle. The text section summarises the laboratory’s result compared to the Mean for Comparison. Laboratory performance for the current cycle is also compared to that of the previous cycle. A variety of charts including Levey-Jennings, Target Score, % Deviation by sample and % Deviation by concentration are also presented allowing a visual assessment of laboratory performance over the cycle.
A certificate of performance is provided listing all parameters, for which the laboratory achieved an acceptable level of performance (Average Absolute SDI <2). Although the End-of-Cycle Report is issued for all registered parameters, the certificate of performance will only be available for parameters where results for at least 50% of samples in the cycle have been returned.
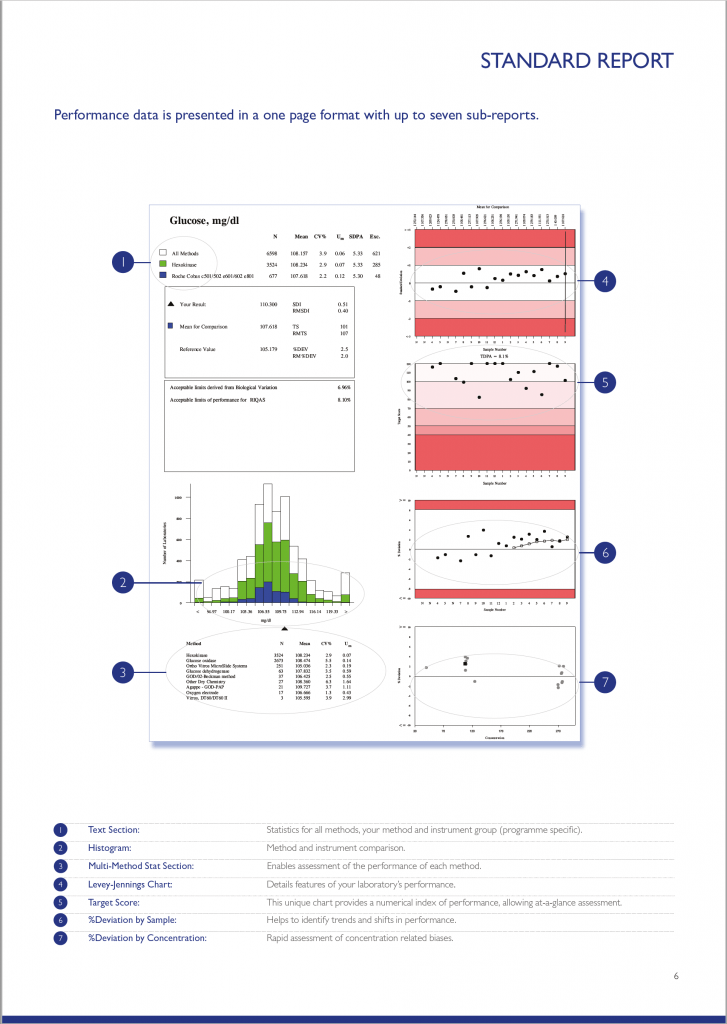
RIQAS Standard Report
There are a variety of RIQAS reports designed to enable quick and easy identification of any trends or test system issues. The standard quantitative report is provided in a user-friendly, one page per parameter format allowing a visual, at-a-glance assessment of performance. The standard quantitative report is split into several easy to interpret subsections each designed to save valuable laboratory time.
You can explore each of the report sections using the table below. Don’t forget, to enlarge the image, simply click on it.
RIQAS EQA Reports
RIQAS Reports Features
The text section provides a statistical breakdown of results by all methods, your method and instrument group. The Mean, CV% and Uncertainty or Measurement is presented for each comparison group.
Your laboratory’s result is compared to the Mean for Comparison (usually the instrument group Mean). Also included are the RIQAS performance indicators; SDI, Target Score and %Deviation.
Acceptable performance criteria:
- SDI <2
- Target Score >50
- %Deviation
The defined acceptable limits default to the RIQAS TDPA values but may be based on CLIA, biological variation or country specific limits.
Performance goals based on Biological Variation are also stated within the text section for information purposes.
The histogram chart provides an overview of how your laboratory’s result compares to the all method group, your method group and your instrument group. Your result is represented by a black triangle; the closer to the centre the better.
The chart is intended to provide a quick visualisation of performance compared to other method groups and can be used to identify any potential bias.
The Levey-Jennings chart plots the last 20 SDI’s and is extremely useful for monitoring EQA performance over time, allowing quick and easy identification of any trends or bias. The chart is colour coded making interpretation simple and easy; results that fall in the white area are excellent and those in the red area unacceptable.
The Target Score (TS) chart is a unique chart which displays your laboratory’s last 20 target scores delivering an instant, visual indication of performance. The TS chart is conveniently colour coded for even easier performance assessment, a TS >50 is acceptable. The TS is a numerical index relating your %Deviation from the mean to a Target Deviation for Performance Assessment (TDPA).
The %Deviation by sample chart displays the %Deviation for the last 20 EQA samples enabling identification of trends and shifts in performance. Similar to the other charts on the RIQAS report, the %Deviation by sample chart is shaded to indicate the limit of acceptable performance. A black dot within the white section of the chart will represent results with a %Deviation within your acceptable limits of performance; a black dot within the red section of the chart will represent results with a %Deviation outside your acceptable limits of performance. %Deviations are not influenced by the performance of your peers, as seen with the standard deviation index (SDI) and therefore is a better indicator of individual performance.
The %Deviation by concentration chart enables easy detection of possible concentration related biases. Unlike the other charts provided on the report, the %Deviation by concentration chart displays the concentration range of the previous 20 samples along the bottom of the chart.
Using this scale along with the percentage deviation, you are provided with a rapid assessment of your %Deviation in relation to the concentration of the:
• Current sample (represented by a square)
• Your 19 previous results (represented by circles)
This chart provides an easy interpretation of potential positive or negative biases at high or low concentrations, or whether a particular sample is a random outlier.
Located at the back of the standard quantitative report, our quick reference summary page details the performance for each registered parameter in the programme.
Within the performance column, RIQAS provides an effortless method of assessing the performance of each parameter within the sample distribution. When a red triangle appears next to the parameter, this indicates that all performance indicators (SDI, TS and %DEV) have exceeded the performance criteria.
The performance indicator limits for each parameter are exceeded when your result produces:
• An SDI greater than +/- 2 standard deviations.
• A Target Score less than 50 (only when Target Scoring is available)
• A %Deviation greater than your set acceptable limits of performance.
A summary CSV file is available on request to all participating laboratories. The report provides a summary of all statistics, acceptable limits and performance indicators as a .csv file for each sample in the cycle.
A retrospective statistics summary is also available, four weeks after the final submission date for parameters where a result was not submitted on time.