RIQAS Point of Care EQA
RIQAS Point of Care EQA
Designed to improve the quality of Point of Care Testing (POCT) in locations such as pharmacies, GP surgeries, hospital out patient departments, sports clinics, supermarkets, diagnostic/treatment and walk-in centres.
RIQAS Point of Care EQA provides independent evidence of the accuracy and reliability of test results.
Why RIQAS Point of Care?
Programme Offering
Tests
Role
Matrix
Lipids (Total Cholesterol & HDL Cholesterol)*
- Risk Factor for heart Disease
- Monitoring lipid lowering therapy
Whole Blood
HbA1c (Glycated Hemoglobin)*
- Diagnosing diabetes mellitus
- Monitoring treatment
- Encouraging self-management
Whole Blood
CRP (C Reactive Protein)*
- Early detection of infectious disease
- Identifying need for antibiotic treatment
Whole Blood
Glucose / Ketones
- Diagnose and monitor diabetes
- Monitor the presence of hypoglycemia (low blood glucose) and hyperglycemia (high blood glucose)
- To determine whether excessive ketones are present in the blood, to detect diabetic ketoacidosis (DKA)
Serum
International Normalised Ratio (INR)
- Used to measure the effect of anticoagulant drugs such as warfarin
- Help diagnose a bleeding disorder; to help estimate the severity of liver disease
Whole Blood
Key Cycle Dates
RQ9181 Distribution Month Sample Distributed Result Submission Deadline January 2024 8th January 17th January February 2024 5th February 14th February March 2024 4th March 13th March April 2024 2nd April 10th April May 2024 6th May 15th May June 2024 3rd June 12th June July 2024 1st July 10th July August 2024 5th August 14th August September 2024 2nd September 11th September October 2024 7th October 16th October November 2024 4th November 13th November December 2024 2nd December 11th December What Participants Say
What participants say
Our unrivalled commitment to quality and service ensures high levels of customer satisfaction, this is evident from the responses to our latest customer satisfaction survey:
“All in all a quick and efficient service”
“Good online system”
“Very helpful team”
“Excellent training”
“They are an experienced team”
“Very satisfied with the service that we receive”
“Very good value for money”
“The website is great”
The Importance of Quality Assurance
Importance of quality assurance
Quality assurance is an essential aspect of any clinical/diagnostic testing service and is aimed at ensuring the accuracy and reliability of patients’ results. The right result allows the right clinical advice to be offered in a timely manner. Quality assurance operates at two levels:Internal Quality Control
Internal Quality Control includes operator training/ competency assessment, analyser/ test system maintenance, and adherence to policies/ processes. Whilst some point of care analysers include inbuilt quality checks, cross-check analysis against samples with known levels provides immediate assurance and evidence that a patient’s result is safe to report.External Quality Assessment
External Quality Assessment involves analysis of samples with unknown levels that have been distributed by an external organisation. Participants are informed how their results compare with other participants, hence providing independent evidence of performance. Increasingly, participation in an external quality assessment scheme is becoming a mandatory requirement where health and healthcare services are being provided.
EQA provides assurance to both staff and customers that testing provides accurate and reliable results.
Want to know more?
Contact us or download the RIQAS Point of Care catalogue to learn more.
Continue Reading
Frequently Asked Questions
RIQAS
Contact Us
Neonatal Bilirubin External Quality Assessment
The RIQAS Neonatal Bilirubin EQA programme has been designed to assess the performance of total and direct bilirubin assays with levels tailored to neonatal bilirubin testing.
- Lyophilised for enhanced stability
- Monthly reporting
- Human based serum
- Submit results and view reports online via RIQAS.net
- Rapid turnaround of reports allows for any necessary corrective actions to be taken with minimal disruption to laboratory output
Not accredited to ISO/IEC 17043
| Cat No | Kit Size | Frequency | Cycle Start | Parameters | |
|---|---|---|---|---|---|
| RQ9191 | 2 x (6x3ml) | Monthly | July | 2 | |
Parameter
- Direct Bilirubin
- Total Bilirubin
Please note, product availability may vary country to country.
Randox International Quality Assessment Scheme (RIQAS)
RIQAS is the world’s largest External Quality Assessment scheme with more than 75,000 laboratory participants spanning over 138 countries.
Key Benefits of RIQAS
Cost Effective
Consolidate EQA analysis, reduce workload and costs with our comprehensive, multi-parameter programmes.
Flexible Programme Options
Flexible programme options are available to suit all laboratory budgets. The option to register up to five instruments per programme (volume permitting) at no extra cost for comparative performance assessment
Accredited
RIQAS is accredited to ISO/IEC 17043:2010 “Conformity Assessment – General Requirements for Proficiency Testing” which is accepted by national and international accreditation bodies. Please see accreditation schedule for additional information.
Expansive Peer Group Database
The availability of large peer groups ensures robust data is available for a wide range of instruments and methods.
Rapid, User-Friendly Reports
Our rapid, user-friendly reports allow at-a-glance performance assessment. Complimentary multi-instrument, inter-laboratory and end-of-cycle reports are also available.
Commutable Samples
A commutable sample matrix increases confidence that performance mimics that of patient samples.
Early Identification of Test System Errors
Frequent reporting allows early identification of test system errors, while our 72 hour report turnaround enables corrective action to be taken with minimum disruption to the lab.
Convenient Data Upload System
RIQAS.net is a convenient, web-based data entry system which allows participants to return results and view reports online.
High Quality Samples
EQA samples are manufactured to the highest quality standard and span clinically relevant levels.
RIQAS EQA Programmes
Choice & flexibility are guaranteed with our 36 programme portfolio.
Browse the programmes below
RIQAS EQA Reports
User-friendly, one-page per parameter reports allow for at-a-glance performance assessment.
Browse the reports below.
What Does RIQAS Provide?

RIQAS Calendar 2024
What the Participants say:
“EcoScense LLC joined RIQAS in 2015 and currently run the General Clinical Chemistry, Haematology and Microbiology Programmes. We chose RIQAS as it is the best quality and has the most accessible programmes with weekly and monthly reporting available. We are very satisfied with the service and support we receive from the Randox team.”
EcoSenseLLC – Aremnia
“Alfa Diagnostica have been participating in RIQAS for 6 years. Over this time, the number of programmes has increased from 4 to 12, including Haematology, Immunoassay, Coagulation and Urinalysis. We chose RIQAS because of its quality, well-thought-out programmes, convenient scheduling and the large number of participants. RIQAS has enhanced the quality of our services by providing a flexible, convenient and objective instrument for self-evaluation. It has served as proof of reliable results for any interested third party.”
Alfa Diagnostica SRL – Republic of Moldova
Continue Reading
RIQAS Brochure
RIQAS Parameters
RIQAS.net
RIQAS Calendar
Contact Us
RIQAS Point of Care EQA
Designed to improve the quality of Point of Care Testing (POCT) in locations such as pharmacies, GP surgeries, hospital out patient departments, sports clinics, supermarkets, diagnostic/treatment and walk-in centres, RIQAS Point of Care EQA provides independent evidence of the accuracy and reliability of test results. Randox International Quality Assessment Scheme (RIQAS) is the world’s largest EQA scheme with over 55,000 participants in more than 134 countries.
Why RIQAS Point of Care?
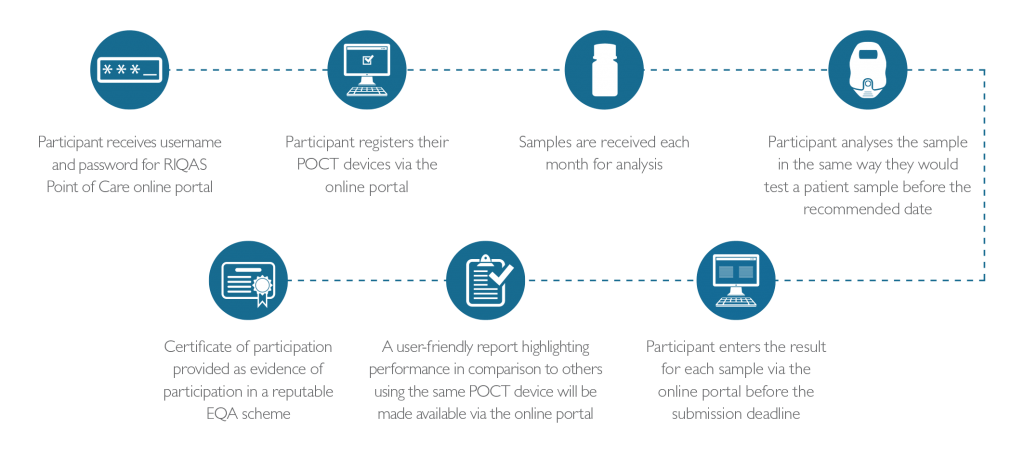
About RIQAS Point of Care
How it Works
Tests and Analysers
Test Role Matrix Lipids (Total Cholesterol & HDL Cholesterol) • Risk factors for heart disease
• Monitoring lipid lowering therapy
Whole Blood HbA1c (Glycated Haemoglobin) • Diagnosing diabetes mellitus
• Monitoring treatment
• Encouraging self-management
Whole Blood C-Reactive Protein (CRP) • Early detection of infectious disease
• Identifying need for antibiotic treatment
Whole Blood Glucose/Ketones • Diagnose and monitor diabetes
• Monitor for the presence of hypoglycaemia
(low blood glucose) and hyperglycaemia (high blood glucose)
• To determine whether excessive ketones are present in the blood, to detect diabetic ketoacidosis (DKA)
Serum International Normalised Ratio (INR) • Used to measure the effect of anticoagulant
drugs such as warfarin
• Help diagnose a bleeding disorder; to help
estimate the severity of liver disease
Plasma Note – The RIQAS Point of Care range is constantly expanding to include new tests and analysers. Please contact us if your desired analyser or test is not currently displayed.Ordering Information
Panel Catalogue Number Lipids RQ9181/A Lipids + 1 panel RQ9181/B Lipids + 2 panels RQ9181/C Additional Sample RQ9181/D Glucose and Ketones RQ9188 INR RQ9189 Pipette Tips RQ9182 Bulbous pipette RQ9183 What Participants Say
Our unrivalled commitment to quality and service ensures high levels of customer satisfaction, this is evident from the responses to our latest customer satisfaction survey:
“All in all a quick and efficient service”
“Good online system”
“Very helpful team”
“Excellent training”
“They are an experienced team”
“Very satisfied with the service that we receive”
“Very good value for money”
“The website is great”Key Cycle Dates
RQ9181 Distribution Month Sample Distributed Result Submission Deadline January 2024 8th January 17th January February 2024 5th February 14th February March 2024 4th March 13th March April 2024 2nd April 10th April May 2024 6th May 15th May June 2024 3rd June 12th June July 2024 1st July 10th July August 2024 5th August 14th August September 2024 2nd September 11th September October 2024 7th October 16th October November 2024 4th November 13th November December 2024 2nd December 11th December Importance of Quality Assurance
Quality assurance is an essential aspect of any clinical/diagnostic testing service and is aimed at ensuring the accuracy and reliability of patients’ results. The right result allows the right clinical advice to be offered in a timely manner. Quality assurance operates at two levels:Internal Quality Control
Internal Quality Control includes operator training/ competency assessment, analyser/ test system maintenance, and adherence to policies/ processes. Whilst some point of care analysers include inbuilt quality checks, cross-check analysis against samples with known levels provides immediate assurance and evidence that a patient’s result is safe to report.External Quality Assessment
External Quality Assessment involves analysis of samples with unknown levels that have been distributed by an external organisation. Participants are informed how their results compare with other participants, hence providing independent evidence of performance. Increasingly, participation in an external quality assessment scheme is becoming a mandatory requirement where health and healthcare services are being provided.
EQA provides assurance to both staff and customers that testing provides accurate and reliable results.Want to know more?Visit our Importance of EQA page to learn more.
Want to know more?
Contact us or download the RIQAS Point of Care catalogue to learn more.
Continue Reading
Frequently Asked Questions
RIQAS
Contact Us
RIQAS Past Panels
RIQAS (Randox International Quality Assessment Scheme) is the largest global EQA scheme with over 50,000 participants in more than 139 countries. Our range currently comprises 36 programmes and the majority of clinical testing.
What are RIQAS Past Panels?
Available Panels
• Ammonia/ Ethanol
• Anti-Müllerian Hormone (AMH)
• Anti-TSH Receptor
• Blood Gas
• BNP
• Cardiac
• Cardiac Plus
• Cerebrospinal Fluid (CSF)
• Coagulation
• CO-Oximetry
• CYFRA 21-1
• Cytokines
• ESR
• General Clinical Chemistry
• Glycated Haemoglobin (HbA1c)
• Haematology
• Human Urine
• Immunoassay
• Immunoassay Speciality 1
• Immunoassay Speciality 2
• Immunosuppressant
• Lipid
• Maternal Screening
• Microbiology (Bacterial Identification)
• Neonatal Bilirubin
• Serology (Anti-SARS-CoV-2)
• Serology (EBV)
• Serology (HIV/ Hepatitis)
• Serology (Syphilis)
• Serology (ToRCH)
• Serum Indices
• Specific Proteins
• Sweat Testing
• Therapeutic Drugs
• Urinalysis
• Urine Toxicology
*Product availability is dependent on RIQAS stock surplus levels.
Benefits of RIQAS Past Panels
All RIQAS Past Panels are provided with an Excel document containing supporting data for the instrument and method of interest.
RIQAS Past Panel samples span multiple levels, making them ideal for ensuring accurate instrument performance across the measuring range.
RIQAS Past Panels allow laboratories to receive comprehensive external QC reports even after program close.
Protected: QCMD – Molecular External Quality Control (NEW)
Point of Care Testing (POCT) Explained

Point of Care Testing (POCT) is the delivery of a test at the point in time at which the result will be used to make a decision and taking appropriate action resulting in an improved health outcome. It is also known as near patient, bed-side, extra-laboratory, decentralised, and ancillary testing [1]. It has been shown to reduce hospital stay time, reduce complications, and improve adherence to treatment [2].
Point of care testing is not a recent practice; many early diagnostic tests were administered at the bedside. However, analytical technology has progressed and multiple tests can be performed within minutes in a laboratory. Recently, this technology has been put into the hands of the staff near the patients [2]. There are two types of technology, benchtop analysers and hand held devices. Bench top systems are just smaller versions of laboratory analysers but some steps are automated. Hand held devices are simple in appearance but complex internally, they can manage several tasks including, adding reagents, separating cells from plasma, and reading colour or other measures.
Results can be obtained faster, allowing for more immediate decisions meaning treatment can begin sooner. Patients can live a longer and higher quality life, helped by a reduction in the length of hospital stays.
Some benefits of POCT [2]:
Key objective
The main objective of Point of Care Testing is to generate results more quickly so that appropriate treatment can be provided, resulting in an improved patient outcome.
Management
Accurate and reliable results can only be obtained if the patient and sample are treated correctly. Point of care testing is likely to be performed by staff with a limited technical background, so training and quality control are vital.
Outcomes
Proper analysis technique alone is not enough to ensure an accurate decision; any test will only be beneficial if the appropriate action is taken based on the result. The effectiveness of POCT is assessed in terms of the overall outcome of the patient.
There are three phases in the POCT cycle: pre-analytical phase, analytical phase, and post-analytical phase. About 90% of quality issues are attributed to the pre-analytical and post-analytical phases [3]. These errors are mainly attributed to user error and can be caused by a number of issues including, selecting the wrong POCT device, not following manufacturer instructions, inadequate training, not adhering to appropriate QC practices, and many more.
The errors can usually be mitigated by implementing an action plan and ensuring it is executed exactly as designed, deviation from the action plan will lead to errors. Errors in POCT diagnostics can lead to misdiagnosis, improper treatment, costly follow-up procedures, and death.
Some strategies for improvement:
Internal Quality Control and External Quality Assessment is conducted to monitor the stability of the analytical measurement system and to alert the operator to a change that may lead to a medically significant error [6].
A study by Price, Smith and Bruel [8] was conducted on a number of labs over a period of time of up to 15 years. They discovered that test result performance improved with time and was associated with regular participation in External Quality Assessment (EQA) schemes and with the use of internal quality control (IQC) procedures.
Internal Quality Control
Internal Quality Control (IQC) is used to assess the day-to-day consistency of assay performance, providing quality assurance for patient results. IQC activities are among the ten most common POCT deficiencies. These may include performing and documenting quality control testing and taking the correct action for outliers [4]. This poor performance could be attributed to how IQC is viewed in POCT; users may lack appreciation of the potential for errors and may see the analyser as infallible, they likely see IQC as an additional workload as opposed to part of their testing routine.
CLSI regulations require risk assessment for each stage of patient testing alongside an implementation of a quality control plan. Below are some suggestions for how IQC should be conducted for POCT.
Conduct
IQC should be conducted when: a new lot of consumable is used; a patient result is queried; after maintenance; the device has been physically insulted. IQC should be conducted by the usual device operator so assurance can be provided for the whole testing process.
Training
ISO 22870 requires POCT users should be trained in the theory and practice of IQC [5]. Staff should be trained in every aspect of POCT including storage, preparation, frequency, documentation and basic troubleshooting.
Material
QC material for POCT should be obtained from a third party provider and not rely on material provided by the device manufacturer, the benefits of which are well documented. It should also contain analytes at clinically relevant concentrations, be provided ready-to-use, and be stable at ambient temperatures.
Results
All IQC results must be recorded with the date, time, user, decision to accept or reject, and any actions taken as appropriate. Locally assigned ranges alongside analyte-specific rules should be used to maximise error detection. An example of how IQC could be recorded and an action flowchart can be seen in Fig. A below.
Troubleshooting
There should be a protocol for required actions following a failed IQC. Any troubleshooting should be developed with knowledge of the most common errors and user capability.
Review
A monthly review should be conducted to identify persistent failures and trends.
The cost of IQC may also be a factor in resistance to IQC, however, while it is difficult to quantify, the cost of not conducting it may be greater in terms of human harm. A whitepaper is available detailing IQC in POCT (download).
Fig. A Examples of a manual IQC documentation, adapted from the Australian Government’s POCT General Practice [4]. (Click to expand)
External Quality Assessment
External Quality Assessment (EQA) or Proficiency Testing (PT) involves running blind patient-like samples and comparing your results to peer results, in order to retrospectively monitor the accuracy of reporting. EQA samples should be treated as if they were a patient sample and therefore must be run by personnel who would normally use the device. This provides confidence in the reliability of patient test results. (Learn more about EQA)
Benefits of participation in an EQA programme include assessment of result accuracy, assessment over time, comparisons with instruments, methods and peers, and providing confidence in test results.
EQA for POCT is, in theory, similar to EQA in a large laboratory. There is a significant difference however, the POCT participants are usually health care professionals with little knowledge of laboratory medicine. A lack of understanding of the importance of EQA had led to a smaller percentage of sites participating than large laboratories.
A Good EQA Scheme
A good EQA scheme should offer:
Conducting EQA in POCT
Below are some suggestions for how EQA should be conducted for POCT.
Conduct
EQA should be conducted by the operator who normally conducts patient testing to ensure the true workflow is assessed [6].
Material
EQA samples should be commutable, meaning they have the same numeric relationship between measurements procedures as is observed for a panel of patient samples (reacts the same as a real patient sample).
Report Frequency
Challenges / surveys should be frequent enough to identify systematic errors in a timely manner, affecting the fewest patient results [10].
Report Turnaround
A fast turnaround time allows test system errors to be identified sooner and necessary corrective actions to be taken immediately with minimum disruption to the lab.
Review
A regular review of past EQA results should be part of the cycle of quality.
Guidance
A POCT EQA provider should be able to provide assistance when the user is having difficulties.
Results
Individuals carrying out testing should have the correct knowledge to interpret results, choosing a scheme with easy to interpret results can help.
Internal Quality Control
Randox offer a number of controls suitable for Point of Care Testing applications:
Acusera Blood Gas Control
The Randox Acusera Blood Gas Quality Controls contain assayed target values for ten parameters, covering pH, pCO2, pO2, electrolytes, glucose and lactate. The material is provided in easy to open ampoules for added convenience and ease-of-use. The liquid ready-to-use nature of the control makes it ideal for use in point-of-care testing and on a wide range of blood gas instruments.
Acusera Liquid Cardiac Control
The Randox Acusera Liquid Cardiac control is designed to be both convenient and easy to use. The liquid ready-to-use format makes it ideal for both clinical laboratories and point-of-care testing. Assayed, instrument specific values are provided for an impressive 8 cardiac markers including, NT-ProBNP, D-dimer and Troponin ensuring consolidation and flexibility. Furthermore, an open vial stability of 30 days for all analytes helps to keep waste and costs to a minimum.
Acusera Liquid HbA1c Control
Liquid Urine Control
The Randox Acusera Liquid Urine quality control is designed to be both convenient and easy to use. The liquid ready-to-use format eliminates issues with pipetting and allows convenient storage at 2℃ – 8℃. Assayed instrument and method specific target values and ranges are provided for 18 commonly tested urine chemistry parameters.
External Quality Assessment
Randox offers RIQAS Point of Care, a simple EQA scheme designed for use in point of care settings. It is a single sample, single scheme programme featuring whole blood samples for authentic patient sample assessment.
RIQAS
RIQAS Point of Care
Acusera
[1] C. Price, A. St john and J. Hicks, “Point-of-care testing”, 2004. [Online]. Available: http://mldt.hu/upload/labor/document/PRICEP.pdf. [Accessed: 23- Jul- 2018].
[2] C. Price, “Point of care testing”, BMJ, vol. 322, pp. 1285-1288, 2001.
[3] A. Okorodudu, “Optimizing accuracy and precision for point-of-care tests”, Acutecaretesting.org, 2011. [Online]. Available: https://acutecaretesting.org/en/articles/optimizing-accuracy-and-precision-for-point-of-care-tests. [Accessed: 24- Jul- 2018].
[4] H. Holt and D. Freedman, “Internal quality control in point-of-care testing: where’s the evidence?”, Annals of Clinical Biochemistry, vol. 53, no. 2, pp. 233-239, 2016.
[5] “ISO 22870:2016 – Point-of-care testing (POCT) — Requirements for quality and competence”, Iso.org, 2018. [Online]. Available: https://www.iso.org/standard/71119.html. [Accessed: 25- Jul- 2018].
[6] J. Gill and M. Shephard, “The Conduct of Quality Control and Quality Assurance Testing for PoCT Outside the Laboratory”, Clin Biochem Rev., vol. 31, no. 3, pp. 85-88, 2010.
[7] A. Stavelin and S. Sandberg, “Essential aspects of external quality assurance for point-of-care testing”, Biochemia Medica, pp. 81-85, 2017.
[8] C. Price, I. Smith and A. Van den Bruel, “Improving the quality of point-of-care testing”, Family Practice, vol. 35, no. 4, pp. 358-364, 2017.
[9] “ISO 15189:2012 – Medical laboratories — Requirements for quality and competence”, Iso.org, 2018. [Online]. Available: https://www.iso.org/standard/56115.html. [Accessed: 31- Jul- 2018].
[10] J. Crilly, “Mythbusting: Frequency of EQA Reports”, Randox Laboratories, 2017.
[11] G. Kristensen and P. Meijer, “Interpretation of EQA results and EQA-based trouble shooting”, Biochemia Medica, pp. 49-62, 2017.
Importance of External Quality Assessment (EQA)

External Quality Assessment (EQA) / Proficiency Testing (PT) allows for a comparison of a laboratory’s testing procedures to other laboratories across the world. Comparisons can be made to a peer group of laboratories or to a reference laboratory.
EQA involves running blind patient-like samples, comparing your results to peer results, in order to retrospectively monitor the accuracy of reporting. EQA samples should be treated as if they were a patient sample and therefore must be run by personnel who would normally use the device. This provides confidence in the reliability of patient test results.
Participating in an EQA scheme allows a laboratory to gather valuable data, this data can be used in a variety of ways [1]:
EQA provides assurance to both staff and customers that testing taking place at your laboratory provides accurate and reliable results. Problems can be identified early on and corrective action can be untaken. The reliability of methods, materials, and equipment can be evaluated and training can be developed and its impact monitored.
Large laboratory groups can compare their performance with sites across their group, ensuring accuracy and consistency no matter where testing takes place.
EQA participation is often a requirement for accreditation, gaining accreditation alone has a host of benefits, not least an increased confidence in results from customers, current and potential.
Read more about accreditation: ‘The Importance of Meeting ISO 15189 Requirements’.
Point of care testing (POCT) refers to testing that is performed near or at the site of a patient with the result leading to a possible change in the care of the patient. The popularity and demand for POCT has recently seen rapid growth, this comes from the advantages including the added convenience of being able to obtain a rapid result at the patient’s bedside, thus allowing immediate action, saving time and improving the potential outcome for the patient.
Although there are many benefits of using POCT devices in terms of their convenience, these benefits are only true if the results produced are both accurate and reliable. Ensuring accuracy and reliability is the primary responsibility of Quality Control.
EQA is strongly recommended for all point of care devices and is recommended by ISO 22870, which providesspecific requirements applicable to point-of-care testing and is intended to be used in conjunction with ISO 15189.
There are many External Quality Control schemes that come in different varieties. EQA schemes can be mandatory, required either by accreditation or law. Others are voluntary and carried out by laboratories who want to ensure that they are carrying out accurate testing and improve the quality of the lab’s performance [1].
A good EQA scheme should offer:
EQA is a great tool for comparing against a peer group and maintaining an effective QC strategy, however, it has its limitations.
EQA / PT alone cannot provide a complete evaluation alone; it is important to also run third party controls regularly. You can find out about the importance of third party controls here.
EQA results can also be affected by variables not relating to patient samples, including preparation, clerical functions, matrix effects, and selection of method. The errors can appear to be a downside to EQA but it can be used as a way to evaluate staff performance as well as assay performance.
If possible, every laboratory should participate in an EQA scheme that covers all testing procedures. Laboratories need to develop a management process with the objective to assure that EQA samples are treated appropriately and in the same manner. This includes, sample handling, sample analysis, record keeping, investigating deficiencies, taking corrective actions, and communicating results with laboratory staff and management.
Problems at any stage of sample analysis can cause errors, when an error does happen, all elements of the process need to be checked. Some examples of errors:
Pre Analysis
• Incorrect sample handling during preparation, shipping or storage
• Improper storage
• The material has expired
• An error in manufacturing
Analysis
• Instrument, calibration or reagent defects
• Staff competency
• Matrix effects
• Incorrect analysis method
Post Analysis
• Report misinterpretation
• Clerical or transcription errors
• Failure to take corrective action
• Where possible, all laboratories should participate in an EQA scheme for all tests that they perform.
• EQA samples should be treated in the same way as a patient sample, using the same procedures, instruments, methods, and staff who normally perform the testing.
• EQA provides valuable resources and data to effectively maintain accurate and reliable results and should be seen as educational.
Randox offers RIQAS, the largest EQA scheme in the world with over 45,000 participants across 133 countries, offering 33 consolidated programmes. Randox also offers a range of over 90 molecular programmes for infectious disease testing with Quality Control for Molecular Diseases (QCMD).
Features and Benefits



Fully accredited to ISO/IEC 17043:2010



Consolidated programmes



High frequency reporting



The highest quality material



User-friendly reports
To learn more about RIQAS, visit the RIQAS homepage.
RIQAS
RIQAS Point of Care
QCMD
[1] WHO, Overview of External Quality Assessment (EQA). World Health Organisation, 2009.
[2] ISO 15189:2012 Medical laboratories — Requirements for quality and competence, 3rd ed. ISO, 2014.
[3] ISO 22870:2016 Point-of-care testing (POCT) — Requirements for quality and competence, 2nd ed. ISO, 2016.
RIQAS Point of Care – FAQs


RIQAS Point of Care
RIQAS
Contact Us
RX series (Concept 3)


The RX series range of clinical chemistry analysers includes both semi-automated and fully automated testing for a range of clinical settings. With a world leading test menu comprising of routine chemistries, specific proteins, lipids, therapeutic drugs, drugs of abuse, antioxidants and diabetes testing, the RX series offers laboratories the complete clinical chemistry package and results you can trust. The RX series was built with three core values in mind – Reliability, Accuracy and Precision.


Consolidation of Routine & Specialised Testing on One Single Platform
With an extensive product portfolio covering over 100 disease markers within routine and nice testing, the RX series removes the need for a separate nephelometry system for specific proteins and allows laboratories to bring all testing in-house; thus ensuring minimal downtime and providing real cost savings through consolidation.
![]()
![]()
Low Reagents & Sample Volumes
Built with excellence in mind, the RX series range of analsyers require a low sample volume to deliver consistent high quality results which is beneficial when working with paediatric patients and animals. Combined with our high quality reagents, the RX series reduce the possibility of misdiagnoses, offering accurate, reliable and precise results each time, every time.


Unrivalled Customer Support
Our team of trained engineers are on hand to work with you in preserving the continuity of your operations while maximising the potential of your RX series instrument. We know time is critical in any laboratory and our global network means we are uniquely positioned to meet your needs with local service and support whenever you need it.









![Fig. A Fig. A Examples of a manual IQC documentation, adapted from the Australian Government’s POCT General Practice [4]](https://www.randox.com/wp-content/uploads/2018/08/Fig.-A.jpg)





