QC Material Stability – Dig a Little Deeper
QC Material Stability
Stability has a number of different definitions, however, the most relevant to clinical diagnostics, and indeed quality control sera, is the “resistance to chemical change or physical disintegration”. Much like a chain, your quality control system is only as strong as its weakest link, or in this case analyte.
Whilst we appear to be stating the obvious here, this might not be as straightforward as it first appears. The product literature you peruse will help you decide what control best suits your needs, whilst many companies will state their control stability in the literature there are some instances where all may not be as it first appears. It is also important to note that some manufacturers may not make stability claims for some of the analytes listed in their control material. In such instances, you are required to validate these in-house, taking up precious time and resources.
Dig a Little Deeper
Whilst we understand that some analytes do have limitations due to their inherent nature, misleading analyte claims can cost the laboratory both time and money. In a recent survey conducted by Randox, 65.5% of respondents indicated that they felt stability was a ‘Very Important’ QC feature. As such it’s important that you look beyond the sales literature when it comes to control stability. Look out for exceptions in the small print of the control kit inserts. For example, if a control has a stability claim of 7 days at 2-8oC and a routine analyte like Cholesterol has a stability claim of just 2 days at 2-8oC then the true stability of the control is only 2 days. In such instances, there is a lot of potential for waste, as laboratories will be required to prepare a new vial of QC material every 2 days leading to increased costs and time. However, if you dig a little deeper into the controls and always read the small print, you could avoid such issues.
How can Randox Acusera benefit you?
For more than 30 years Randox has been shaping the future of clinical diagnostics with our pioneering high quality, cost effective laboratory solutions. Quality Control is our passion, we believe in producing high-quality material that can help streamline procedures, whilst saving money for laboratories of all sizes and budgets. We pride ourselves in not misleading our customers with false stability claims for our controls. With controls such as our Liquid Cardiac and Specific Proteins Controls, you could benefit from a 30-day open vial stability for all analytes, without exception.
By employing our Randox Acusera quality control materials you could benefit from;
Commutable controls, ensuring a matrix that reacts to the test system in the same manner as a patient sample, enabling an accurate and reliable assessment of instrument performance.
Accurate target values that won’t shift throughout the shelf life of the controls, eliminating the need to spend valuable time and money assigning values in-house.
Consolidation of test menu with controls comprising up to 100 analytes, reducing preparation time and storage space required.
Analytes present at clinically relevant levels ensuring accurate test system performance across the clinical range, maximising laboratory efficiency by eliminating the need to purchase additional high or low-level controls at extra expense.
True third party controls designed to provide an unbiased assessment of performance, our Acusera controls have not been manufactured in line with or optimised for use with any particular reagent, method or instrument.
For more information on any of our products, or to request a consultation from one of our QC Consultants, contact us via acusera@randox.com.
What is Measurement of Uncertainty?
Measurement Uncertainty (MU) relates to the margin of doubt that exists for the result of any measurement, as well as how significant the doubt is. For example, a piece of string may measure 20 cm plus or minus 1 cm, at the 95% confidence level. As a result, this could be written: 20 cm ±1 cm, with a confidence of 95%. Therefore, we are 95% sure that the piece of string is between 19 cm and 21 cm long.
Standards such as ISO 15189 require that the laboratory must determine uncertainty for each test. However, they have not specified how this should be done.
How do we calculate Measurement Uncertainty using QC data?
Employing your QC data to calculate uncertainty makes several assumptions; your test system is under control, the patient samples are treated in the same manner as your controls and gross outliers have been removed. If you choose to use your QC data to calculate this you should ensure that you use a commutable control with a matrix similar to that of a patient sample, with analytes present at clinically relevant levels
To calculate MU, labs must look at the intra-assay precision and inter-assay precision of their test.
Intra-assay precision: Sometimes known as ‘within run’ precision, is where 20 or more replicates of the same sample are run at the same time, under the same conditions (calculated from a single experiment). Intra-assay precision helps to assess systematic uncertainties
Inter-assay precision: Sometimes known as ‘between run’ precision, is where 20 or more replicates are run at different times – e.g. 1 replicate every day for 20 days (can be calculated from routine IQC data). Inter-assay precision can help identify random uncertainties within the test system.
*The Australian Association of Clinical Biochemists (AACB) recommends that at least 6 months’ worth of QC data are used when calculating the inter-assay precision1.
Once the data is collected, you must calculate the standard error of the mean (SEM) of the intra-assay precision (A) and the SD of the inter-assay precision (B) in order to measure the uncertainty (u). Once A and B have been calculated, they need to be squared, added together and the square root of the sum found:
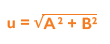
As uncertainty is calculated as SD and 1SD is equal to 68% confidence on a standard Gaussian curve, we can conclude that if we multiply using a coverage factor of 2, we can attain 2SD confidence of 95%. This is known as the Expanded Uncertainty (U):
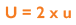
What is the Advantage of Measurement Uncertainty for a lab?
Labs need to carry out MU as it is a requirement of ISO 15189. It states: “The laboratory shall determine measurement uncertainty for each measurement procedure, in the examination phases used to report measured quantity values on patients’ samples. The laboratory shall define the performance requirements for the measurement uncertainty of each measurement procedure and regularly review estimates of measurement uncertainty”.
MU also helps determine whether the difference between two results is negligible due to uncertainty or significant due to a genuine change in condition of the patient; giving labs a greater confidence in reported results.
How can Randox help?
Our new Acusera 24.7 Live Online software provides automatic calculation of MU, saving valuable time and helping labs meet ISO 15189 requirements with ease.
Contact marketing@randox.com to find out how your lab can benefit from Acusera 24.7 Live Online
Take steps to prevent incorrect patient results by making one simple change
According to the NHS Litigation Authority; in 2015 within the UK alone, £193,680,744.30 was spent on ‘wrong diagnosis’ or ‘failed/delayed diagnosis’ causing huge financial strain and impact on labs.
With approximately 75% of clinical decisions and diagnosis based on laboratory test results. The only way to guarantee a high degree of accuracy is to implement a good Quality Control plan. The importance of this is recognised globally, several bodies exist internationally including ISO (International organisation for standardisation) who have developed a set of guidelines and quality systems to ensure the reliability of laboratory test results.
So what can you do to improve accuracy and reliability?
Choose a third party QC
ISO 151589:2012 Section 5.6.2.2 states that “the use of third party control materials should be considered, either instead of, or in addition to, any control materials supplied by the reagent or instrument manufacturer”.
First Party Controls are those manufactured by the instrument/reagent manufacturer. These controls are optimised specifically for use with the manufacturers test system and therefore will mask a multitude of weaknesses. First Party Controls tend to result in perceived accuracy and a biased assessment of performance.
Third Party Controls on the other hand are designed to be completely independent and are not optimised for use with a specific test or system. Leading manufacturers of third party controls will assign target values based on data collected from thousands of independent laboratories, ensuring the availability of statistically robust multi-method, multi-analyser data. Therefore laboratories using Third Party Controls can be assured of unbiased error detection across multiple platforms.
Randox Acusera is a world leading manufacturer of true third party controls providing a cost effective, high quality solution for any laboratory-regardless of size or budget.
Look out for QC samples with clinically relevant concentrations
ISO 15189:2012 states that ‘The laboratory should choose concentrations of control materials wherever possible, especially at or near clinical decision values, which ensure the validity of decisions made’.
It is important to assess the full clinical range of an assay i.e. the range between the lowest and highest results which can be reliably reported. In order to make sure a laboratory instrument is performing accurately across the full clinical range and in particular at the medical decision level, QC materials that cover low, normal and elevated concentrations should be used.
Due to the superior manufacturing process used by Randox, QC target values consistently cover the MDL of tests. By ensuring the controls in use cover clinical decision levels laboratories can be confident of the reliability and accuracy of the patient results they release.
Opt for a commutable control material
A good QC material has many essential properties but above all, controls must perform consistently and reflect the performance of patient samples – if a control meets these requirements then we can say it is commutable. Having a commutable control would aid in the prevention of incorrect patient results because they replicate the performance of a patient sample and react to the test system in a similar manner. Use of a commutable control will also reduce costly shifts in QC target values when reagent batch is changed.
At Randox we take quality seriously, that’s why all QC products are manufactured to the highest possible standard, delivering controls of unrivalled quality. Designed to be commutable, the Acusera range will ensure accurate and reliable instrument performance while simultaneously helping laboratories to meet ISO 15189:2012 requirements. A good QC process will include the use of Third Party Controls, Clinically Relevant Concentrations and controls which can be described as commutable. By employing Quality Control’s that encompass these traits, a laboratory professional can be certain that they have taken the necessary steps to decrease incorrect results and therefore potential misdiagnosis.
Acusera 24•7 Frequently Asked Questions (FAQ’s)

Is it possible to compare multiple instruments on a single Levey-Jennings Chart?
With Acusera 24•7 users can combine multiple instruments, as well as analytes and QC lots on a single chart. This will enable comparative performance assessment and facilitate immediate visualisation of any ongoing or emerging trends. It may also be useful for troubleshooting out of control QC events.
Is it possible to compare different levels of control on a single Levey-Jennings Chart for identification of concentration related bias?
Yes data for multiple QC lots can be displayed on a single, convenient Levey-Jennings chart allowing any concentration related problems to be identified quickly.
How often is peer group data updated?
With Acusera 24•7, peer group data is uniquely updated live in real-time. The instant nature of the peer data will help reduce time and money spent troubleshooting, re-running QC samples and performing any instrument maintenance. With real-time peer group data you can compare to other laboratories around the globe using the same lot of QC material and identify if there are any issues and whether they are unique to your lab or a widespread issue.
Is there a limit to the number of users?
There is no limit to the number of concurrent users – you can have as many as you want. There are 5 different levels of user access – Admin, Group Co-Ordinator, Manager, User and Technical Support. It is worth noting that User access can be customised per user to ensure access to only the required functionality
How can the software help me to meet ISO 15189:2012 requirements?
Unique to Acusera 24.7 , our software will automatically calculate Measurement Uncertainty (UM) and provide your laboratory with a printable report that can be used to help meet ISO 15189:2012 accreditation requirements. In addition to this the software can help to prevent the release of patient results in the event of a QC failure for example when the Quality Control rules are violated.
Is it possible to use RiliBAK as my allowable limits?
QC data can indeed be rejected or alerted based on RiliBAK guidelines. Other options are also available, including, CLIA, Biological Variation, RIQAS TDPA and user defined performance limits.
Is Acusera 24.7 secure?
To authenticate users, a number of security measures are used, including; participant number, username and a password combination (for individual role-based accounts). Password complexity standards are enforced on user account setup. CAPTCHA is enforced after several failed login attempts to prevent or guard against automated attacks. HTTPS and X509 certificate authentication is in place meeting industry security standards.
I have never used interlaboratory software before, is there training provided with my purchase of 247?
There are a number of different options available in terms of training. The easiest, most convenient and accessible form of training is through the use of our walk through demo that has voice-over and text so everyone can follow. With our software comes a user guide, this is a walk through that laboratories can use to guide them through the usage of the software. Additionally, there is also the possibility of a live demo from the sales consultant in your area who will be able to run through the software with you.
Can I create unique login credentials for each user?
Yes, each user will have their own personal log-in. If a lower level user, e.g. user, technical support or manager forgets their password they can have it reset by the administrator. However, if a group co-ordinator or admin forgets their password they should contact Randox directly who will be able to reset their credentials and resend to the administrator’s email address.
I currently use non-Randox Quality Controls, does this affect the ability of the software?
Not at all, our software is so flexible that it can be used with any other manufacturers Quality Control material. However, you will only have access to the internal functions of the software and will not have access to peer group statistics. For this reason we recommend the software is used alongside our Acusera true third party Quality Control solutions.
Is Acusera 24.7 the only software option available?
Yes this is the only option available – Unlike other manufacturers there is no need for any additional software packages or options. All functionality is available in the one software package.
My computer is very old, do I need a new, modern and up-to-date operating system to run the software?
Not necessarily. As long as you have stable access to the internet you can access Acusera 24.7 as it is a cloud based software.
My patient data is confidential. Will the software need access to this data?
Acusera 24.7 will not require access to your patient data. This is important for a laboratory but less for the software. Acusera 24.7 will only need access to the results of your Quality Control to ensure that your instrument/s are performing to standard and therefore ensuring that your patient results will be reliable and accurate.
I have forgotten my username and password – what do I do now?
If an individual with user level or manager level access forgets their username and password, they should contact the laboratory administrator. If an administrator or group co-ordinator forgets their username or password they should contact Randox who will verify the administrator and send new login details for the account.
Is Acusera 24.7 Connect required to import QC data?
Acusera 24.7 Connect is only required if you wish to import QC data automatically. Data can also be entered manually using the data entry screen or in a semi-automated manner using the EDI function.
I need to renew my license, is this done automatically?
If your licence has expired and you would like to renew you should get in touch with your local Sales Consultant.
My internet connection isn’t great. Will this affect the running of the software? What happens if the signal drops when entering results?
If connection is lost from the laboratory’s side, all data will be transferred to the web and once reconnected, the previous session will also be remembered. Emergency power generators and fall over servers are in place to ensure 99.8% uptime is guaranteed.
Are there any additional software requirements?
You must have access to a Java applet. This software is available as standard on almost all modern computers, laptops and notebooks.
Aliquoting for longer QC stability
Al-i-quot: An amount that is an exact divisor of the whole quantity of a substance (Collins Dictionary of Medicine, R. Young, 2005).
Why aliquot QC material?
Aliquoting QC material can extend the open vial stability of a lyophilised control, according to manufacturer recommendations. By splitting your QC material into a number of tubes and freezing these you can extend the working stability of the control, ultimately reducing wastage and the amount of money spent on unnecessary additional controls.
Example
A laboratory purchases a lyophilised QC with a volume of 3ml once reconstituted the control is stable for 7 days at 2-8oC. However, the laboratory only uses 1ml of this control per week, meaning that 2ml could potentially be wasted. The manufacturer states that the control can be frozen after reconstitution, extending the working stability from 7 days at 2-8oC to 30 days at -20 oC to -80oC. The following outlines the process for aliquoting reconstituted material and extending the control’s working stability.
Aliquoting reconstituted material
- Reconstitute the QC material according to the manufacturer’s instructions.
- Using a micropipette aliquot the required volume (generally a minimum of 0.5ml should be used) of reconstituted material into a tube.
- Repeat step 2 until all the reconstituted material has been aliquoted.
- Label each tube with the date the material was reconstituted to avoid the use of expired material.
- Store each aliquot at -20oC in a frost free freezer. Be sure to check the kit insert for frozen stability claims.
- Remove and thaw each aliquot as and when required making sure to use all material within the frozen stability period.
- Once thawed do not refreeze, dispose of any leftover QC material.
Conclusion
Aliquoting reconstituted material is an ideal way of extending the control’s open vial stability. This will ensure that your laboratory minimises the amount of QC material wasted and saves money by eliminating the need to purchase additional controls. Please note that not all lyophilised controls can be frozen like this. To ensure the controls you are selecting are suitable for aliquoting check the product’s kit insert or contact your supplier.
What can Randox Quality Control offer?
We have a number of lyophilised controls which can be prepared and stored in this way across our extensive product portfolio. To find out more visit www.randoxqc.com or contact us via acusera@randox.com to arrange a visit from one of our QC Consultants.
The Benefits of Peer Group Data to your Troubleshooting Process
Drive for more accurate results in your laboratory
We’ve all been there, you’re in the middle of a run of patient tests when you are alerted to an out of control event, such as your analyser is reporting QC results 25% low to target. What do you do? In reality, we all know that the problem is unlikely to correct itself, especially if it’s a calibration or analyser issue. Human error is a potential factor, however all possible causes must be eliminated to proceed with patient testing.
What’s the solution?
ISO 15189:2012 recommends that a laboratory should “have a procedure to prevent patient results in the event of a quality control failure”. Implementing an interlaboratory data management program which features peer group reporting can help you meet this requirement and monitor the results you are producing. Such programs can help detect errors in the analytical phase of patient testing, through the automatic application of pre-programmed QC rules, thus alerting staff to failed results.
Why must Peer Groups be a feature?
A peer group is defined as a “Community in which most or all members have roughly the same characteristics…” (Businessdictionary.com, accessed 2017). In this instance the characteristics could refer to the; instrument, test method or QC material in use. As such peer group programmes could help you detect errors in your laboratory by comparing your results to those who are employing a similar method, instrument and QC to what you are using, i.e. comparing apples for apples. Therefore it is essential that the peer group data you require is available in real-time, to ensure you are accessing the most up-to-date data when reviewing your patient test results.
Scenario
Take the example from the introduction. You’re in the middle of a run of patient tests when you are alerted to an out of control event, such as your analyser is reporting QC results 25% low to target. As part of your troubleshooting procedures, you are able to compare your results to the results of your peer group and note that this is an isolated incident. Consequently, you have eliminated a widespread problem with the QC, reagent or calibrator and narrowed down the root cause to one of the components in your test system. Thus saving you time in the troubleshooting process.
Benefits of Peer Group Comparison
There are a number of benefits to employing peer group comparison in your laboratory. Peer group data comparisons facilitate faster troubleshooting, helping you identify whether the problem you are seeing is unique to your laboratory, or if other laboratories are reporting the same issue. If other laboratories are reporting the same issue it is possible to conclude that there is a widespread problem with either the QC, reagent or calibrator. On the other hand, if it is not occurring within your peer group you will have to investigate further, reviewing your QC processes. As a result, you could resolve issues much quicker by eliminating either a supplier or laboratory issue. Furthermore, you can also eliminate the need for unnecessary repeat tests or instrument maintenance, saving both valuable time and money.
Other characteristics you should look out for
Whilst peer group comparison is a useful feature there are a number of other features you should consider when selecting the right interlaboratory data management program for you. These include;
- Automatic calculation of Measurement Uncertainty, Total Error and Sigma Metrics
- Multiple laboratory management on a single platform
- Accessing data anytime, anywhere via PC, laptop or tablet via a web-based platform
- All data charts you may require to assess whether any bias or imprecision issues are present
- Ability to combine data for multiple QC lots, analytes and instruments on a single Levey-Jennings or Histogram chart
- Automated data import via a direct connection to your LIMS
What can Randox offer?
At Randox we are passionate about quality control and believe in producing high-quality material that can streamline procedures for laboratories of all sizes and budgets through our Randox Quality Control brand. Acusera 24.7 Live Online is just one aspect of our extensive laboratory portfolio that has been designed to help you produce results you can trust. With Acusera 24.7 Live Online you can drive for more accurate results by monitoring and interpreting QC data online, anytime, anywhere. With access to an impressive range of features, including the automatic calculation of Measurement Uncertainty, Total Error and Sigma Metrics, Acusera 24.7 will ensure analytical quality.
Acusera 24•7 Live Online – Speeding up the Review of QC Data
Reviewing QC data can be an extremely time consuming and costly process. With manual statistical calculation laboratories risk missing or ignoring significant trends in QC data which could potentially put patients at risk. So how does a laboratory combat this? Simple; participate in an interlaboratory data management program that provides a quick, effective, accurate and detailed analysis of QC results. The answer to this program is Acusera 24•7 Live Online.
Acusera 24•7 Live Online
With the launch of Acusera 24•7 Live Online version 2.0, QC data review is now faster and simpler than ever before. Our program aims to save the laboratory precious time and money by instantly flagging any QC failures, ultimately ensuring accurate test system performance.
Designed to complement and be used primarily with our Acusera range of true third party controls, Acusera 24•7 Live Online has two primary functions; 1) management and interpretation of IQC data and 2) rapid and effective troubleshooting of QC failures via access to instantly updated worldwide peer group statistics.
These two functions have one common goal – being an effective tool for evaluating laboratory performance. With the launch of version 2.0 the software boasts even more functionality than before, ensuring any laboratory employing Randox Quality Control coupled with Acusera 24•7 Live Online will see benefits from the get-go.
Why should you use Acusera 24•7 Live Online?
Using Acusera 24•7 to help speed up the review process in your laboratory can reap dividends. The program has been designed for this specific reason and the features are geared towards helping the laboratory review, interpret, and analyse QC data quickly, effectively and accurately. One such example of this is the unique dashboard function which instantly flags any alerted or rejected results from the past 7 days, significantly reducing the time spent analysing reports and charts whilst simultaneously allowing any corrective action to be taken immediately with minimum disruption to the lab’s output.
Previously, peer group statistics would have been updated every 24 hours with Acusera 24•7 Live Online version 1.6, however, with the new release, peer data is about to get a unique upgrade. Gone are the days when you will have to wait 24 hours to get updated stats – Acusera 24•7 Live Online now has the ability to generate peer data live in real-time, thereby enhancing the laboratory’s troubleshooting capabilities and allowing labs to compare their data with others around the globe. What’s more there is no deadline for submission of results meaning labs can get a true reflection of performance at any time. Ultimately, laboratories will be able to easily identify if an issue is unique to them or a widespread issue amongst their peers. Such information will allow them establish a root cause quicker and spend less time troubleshooting.
The capacity to generate interactive charts and comprehensive reports automatically is a feature included in Acusera 24•7 that will aid quick review of QC data. Reports can be generated for a user-defined date range and provide a wealth of information. Reports include statistical analysis, statistical metrics, measurement uncertainty, exception and audit trail reports. Reports coupled with Levey-Jennings, Histogram and Performance Summary charts enable rapid and stress-free performance monitoring. The ability to add multiple instruments, QC lots and analytes to a single chart allows for comparative performance assessment and immediate identification of any trends.
We must not forget that Acusera 24•7 Live Online has already had a modernisation in the past few months. In November 2016 we announced the automatic calculation of Measurement of Uncertainty, Total Error and Sigma Metrics. These new features are also included in the version 2.0 launch of our Live Online program.
Our software is highly flexible with custom configurations of performance limits, multi-rules and target values designed to meet and exceed every laboratory’s needs.
With the ability to identify trends, system errors, minimise false rejections and bridge the gap between IQC and EQA, there really is no reason to look elsewhere for your analytical performance of QC.
For more information on Acusera 24•7 Live Online or our Acusera third party controls, click here.
Celebrate Christmas with Randox Quality Control
T’was the week before Christmas and all through the lab not a thing could be heard not even a sound. The analyser lay silent asleep in the corner, the lab staff at home dreaming of a few days’ rest, only a few more days to go before the big day!
The big man in red, what will he bring those who already have everything? Peace, happiness and health for their loved ones throughout the festive break, that would be the wish for everyone to make. And what better way to ensure they stay healthy, well it all begins in the laboratory…
An important consideration to remember when choosing your lab Quality Control (QC) is that approximately 70% of clinical decisions are based on laboratory test results. It is therefore essential that the results gained from laboratory testing are accurate and reliable in order to provide the appropriate treatment and avoid or prevent potential misdiagnosis.
Patient results are of the utmost importance for a laboratory and therefore running the best Quality Control material should be at the top of their agenda. QC material should have a number of features that allow a lab to judge the overall quality of their output. These features include the controls ability to be commutable (which means how well it reacts as a replicate of a patient sample), is it a true third party control that has been manufactured to provide an independent and unbiased assessment of performance, does your control come with clinically relevant levels and does it have a long shelf life as well as a good open vial or reconstituted stability? These are the questions lab staff will be asking themselves when deciding on what QC is the right QC.
So stay off Santa’s naughty list by providing accurate and reliable patient test results, do this by employing Randox QC in your laboratory. Our controls have been designed to deliver significant cost savings without sacrificing on quality. With consolidated controls (combining up to 100 analytes in a single vial) your lab can reduce QC costs and preparation time, the inclusion of analytes present at clinical decision levels will eradicate the need for additional controls and because of our long shelf life (2 years for liquid controls, 4 years for lyophilised) and excellent stability claims your laboratory can be sure that expensive lot changes will be a thing of the past! Our controls can be described as true third party and this, combined with the commutable nature of the controls, leads to us being able to claim that we have the best Quality Control material around.
So this Christmas when deciding what QC to choose – make sure you look no further than Randox Quality Control. Our QC family is known as Acusera and our product offering includes QC and calibrator material, Interlaboratory Data Management Program (Acusera 24.7), the world’s largest international EQA/PT scheme better known as RIQAS and the newest addition to the family, Linearity or Calibration Verification material.
We have packages for every lab regardless of size and budget and we guarantee you will become ho-ho-hooked on Randox QC.
Wishing you all season’s greetings and a prosperous New Year from everyone at Randox QC.

Liquid HbA1c Quality Control

Conveniently supplied liquid ready-to-use the Liquid HbA1c control is ideally suited to both clinical laboratories and POCT helping to significantly reduce preparation time. With a stability of 30 days waste and costs are also kept to a minimum.
Features & Benefits
- Liquid ready-to-use
- Human based whole blood
- Assayed target values
- Convenient bi-level pack covering clinically relevant decision levels
- Stable to expiry date at 2°C – 8°C
- Open vial stability of 30 days at 2°C – 8°C
Description Size Analytes Cat No
Liquid HbA1c Control 2 x 2 x 0.5 ml 1 HA10155
Liquid HbA1c Control Level 1 6 x 1 ml 1 HA10224
Liquid HbA1c Control Level 2 6 x 1 ml 1 HA10225
Analytes
HbA1c (Haemoglobin A1c)
What is Six Sigma?
Six Sigma is a method of process improvement which focuses on minimizing variability in process outputs. The Six Sigma model was developed by Motorola in 1986, and Motorola have reportedly saved over $17 Billion due to its successful implementation.
The model looks at the number of standard deviations (SD) or ‘sigmas’ that fit within the quality specifications of the process. In the laboratory, the quality specifications relate to the Total Allowable Error (TEa). The higher the number of standard deviations that fit between these limits, the higher the sigma score and the more robust the process or method is. As sources of error or variation are removed from a process, the SD becomes smaller and therefore the number of deviations that can fit between the allowable limits is greater; ultimately resulting in a higher sigma score.
A process with a sigma score of six is considered to be a high quality process, making six the target for many industries including the clinical laboratory.
In order to achieve Six Sigma, a process must not produce more than 3.4 defects per million opportunities. In a Laboratory context, this would equate to 3.4 failed QC results per million QC runs.
Sigma is calculated using the following equation:
Sigma = (TEa – %Bias) / %CV
TEa – Total Allowable Error
%Bias – Deviation from the target or peer group mean
%CV – Imprecision of the data
Why is Six Sigma useful in the laboratory?
Six Sigma can be used to help answer one of the most commonly asked questions in laboratory quality control. How often should I run QC?
The Six Sigma model allows laboratories to evaluate the effectiveness of their current QC processes. Its most common use is to help implement a risk-based approach to QC, where an optimum QC frequency and multi-rule procedure can be based on the sigma score of the test in question. The performance of tests or methods with a high sigma score of six or more may be evaluated with one QC run (of each level) and a single 1:3s warning rule. On the other hand, tests or methods with a lower sigma score should be evaluated more frequently with multiple levels of QC and a multi-rule strategy designed to increase identification of errors and reduce false rejections.
The below table shows how multi-rules and QC frequency can be applied according to Sigma Metrics:
| Sigma Score | QC Frequency | Number of QC Samples | QC Rules |
| 6 or more | Once per day | Each level of QC | 1:3s |
| 5 | Once per day | Each level of QC | Multi-rule strategy |
| 4 | At least twice per day | Each level of QC | Multi-rule strategy |
| < 4 | At least four times per day | Each level of QC | Multi-rule strategy |
It is important to note that this is just an example and it may be necessary to run QC samples more often than three times per day. Some high throughput laboratories prefer to run QC samples before and after a set number of patient samples, while others opt to run QC samples after a set period of time. Whatever frequency you choose it is vital that the frequency is appropriate for the test in use. Download our guide ‘How often is right for QC’ to find out more.
What can Randox offer?
Randox’s Acusera 24.7 Live Online is a peer group reporting software application designed to complement the Acusera QC range. The intuitive and user-friendly software boasts some of the most advanced features on the market, and Version 1.6 provides automatic calculation of sigma scores for individual assays, giving the user an at-a-glance overview of assay performance.
Peer group reporting software is an integral part of any modern laboratory seeking to streamline their QC processes and reduce costs. With Acusera 24.7 Live Online, there has never been a better time to implement, save and succeed.
Contact us today to find out how Randox can help your laboratory achieve its goals.







