Committed to meeting customers’ needs
At Randox Quality Control, we strive to meet and exceed customer expectations ensuring high quality products and superior customer service are at the top of our priority list.
How can Randox Quality Control help you?
High Quality QC
The Acusera range of true third party controls boasts an impressive range of benefits ultimately designed to help laboratories reduce costs and time while also ensuring an accurate and reliable test system.
The extended shelf life of our controls allows the same lot of control to be used for a period of up to 2 years keeping costly new lot validation studies to a minimum. We may also be able to sequester lots on your behalf.
The availability of commutable controls designed to react to the test system in the same manner as a patient sample and controls targeted at clinical decision levels will not only help you to meet ISO 15189:2012 requirements but will effectively challenge instrument performance.
Click here to find out more about our QC range.
Customer support
The Randox global support network are on hand with expert advice to ensure timely, accurate and helpful resolution of any issues or queries you may have. The added benefit of quick delivery of product orders further highlights how we work with and for our customers to provide the best service available.
Customer Reviews
Don’t believe us? Read a few of the reviews we have received from laboratories around the world;
“I would like to thank the Randox team for the excellent service when helping to reserve and manage our IQC orders, lot numbers and stock.” – Chief Biomedical Scientist, London, 2017.
Request your free QC consultation by contacting us today! Get in touch and we can arrange for your laboratory to have a consultation with one of our Randox QC specialists. Alternatively, if you would like to leave us a review you can do so by emailing acusera@randox.com.
Product Focus: Calibrators
Calibrators
The importance of accurate instrument calibration in the laboratory is often overlooked. At Randox Quality Control we believe that accurate calibration is the foundation for producing reliable patient results. In fact, we believe that this should be your first step in ensuring accurate instrument performance and something you should consider carefully.
Effective instrument calibration will provide you with a baseline for your analysers from which accurate results can be produced. This baseline is then used to adjust readings to match the computed value and ensure accurate patient results are reported.
Calibrator features you should consider;
As well as frequent recalibration, according to the instructions provided by the instrument / reagent manufacturer, Randox Quality Control recommends;
- Utilising a third party calibrator, such as those available from our Acusera range, which have independently assigned values and have not been optimized for use with any specific instrument or reagent system
- Ensuring the calibrator you employ has the same matrix as a patient sample
- Choosing a calibrator that is 100% commutable, ensuring it mimics the behaviour of a patient sample
- A multi-analyte calibrator with a long shelf life
Clinical Chemistry Calibration Serum
Third party calibrator covering 42 commonly used clinical chemistry tests. Method and instrument specific target values and ranges are provided for most chemistry analysers. Two clinically significant levels are available.
Features & Benefits
- Lyophilised for enhanced stability
- Human based serum
- Instrument specific target values provided for 42 parameters
- Stable to expiry date at 2°C – 8°C
- Reconstituted stability of 7 days at 2°C – 8°C or 4 weeks at -20°C
A week dedicated to unsung heroes! – Medical Laboratory Professionals Week 2017
From April 23rd to April 29th we are celebrating Medical Laboratory Professionals Week! This is a week dedicated to raising awareness for those who work in a laboratory & the hard work that goes unnoticed every day in laboratories around the world.
Have you ever wondered what happens between submitting your patient sample and receiving your results? Have you ever wondered who conducts the detailed laboratory testing for your annual check-up such as cholesterol and glucose levels? Or who analyses these results? The answer, a Medical Laboratory Professional (MLP). MLP’s provide up to 70% of the medical laboratory results for physicians and others to make informed decisions about a patient’s diagnosis and aftercare treatment plan. The work that laboratory professionals do each and every day is integral to providing excellent patient care. They perform and interpret billions of laboratory tests every year.
Providing accurate and reliable test results is of the utmost importance for laboratory professionals and also for us at Randox. With a passion for Quality Control, and with more than 30 years’ experience developing Laboratory QC for the in vitro diagnostics market, we believe in producing high quality material designed to streamline procedures, whilst reducing costs in laboratories of all sizes and budgets. These qualities have been reflected in our Acusera true third party quality controls, Acusera 24.7 interlaboratory data management software, Acusera Verify Calibration Verification material and RIQAS, the largest international EQA scheme.
Randox Quality Control would like to take this opportunity to thank all the laboratory professionals around the world and especially our own laboratory staff – you truly are the “Unsung Heroes of Healthcare”.

QC Material Stability – Dig a Little Deeper
QC Material Stability
Stability has a number of different definitions, however, the most relevant to clinical diagnostics, and indeed quality control sera, is the “resistance to chemical change or physical disintegration”. Much like a chain, your quality control system is only as strong as its weakest link, or in this case analyte.
Whilst we appear to be stating the obvious here, this might not be as straightforward as it first appears. The product literature you peruse will help you decide what control best suits your needs, whilst many companies will state their control stability in the literature there are some instances where all may not be as it first appears. It is also important to note that some manufacturers may not make stability claims for some of the analytes listed in their control material. In such instances, you are required to validate these in-house, taking up precious time and resources.
Dig a Little Deeper
Whilst we understand that some analytes do have limitations due to their inherent nature, misleading analyte claims can cost the laboratory both time and money. In a recent survey conducted by Randox, 65.5% of respondents indicated that they felt stability was a ‘Very Important’ QC feature. As such it’s important that you look beyond the sales literature when it comes to control stability. Look out for exceptions in the small print of the control kit inserts. For example, if a control has a stability claim of 7 days at 2-8oC and a routine analyte like Cholesterol has a stability claim of just 2 days at 2-8oC then the true stability of the control is only 2 days. In such instances, there is a lot of potential for waste, as laboratories will be required to prepare a new vial of QC material every 2 days leading to increased costs and time. However, if you dig a little deeper into the controls and always read the small print, you could avoid such issues.
How can Randox Acusera benefit you?
For more than 30 years Randox has been shaping the future of clinical diagnostics with our pioneering high quality, cost effective laboratory solutions. Quality Control is our passion, we believe in producing high-quality material that can help streamline procedures, whilst saving money for laboratories of all sizes and budgets. We pride ourselves in not misleading our customers with false stability claims for our controls. With controls such as our Liquid Cardiac and Specific Proteins Controls, you could benefit from a 30-day open vial stability for all analytes, without exception.
By employing our Randox Acusera quality control materials you could benefit from;
Commutable controls, ensuring a matrix that reacts to the test system in the same manner as a patient sample, enabling an accurate and reliable assessment of instrument performance.
Accurate target values that won’t shift throughout the shelf life of the controls, eliminating the need to spend valuable time and money assigning values in-house.
Consolidation of test menu with controls comprising up to 100 analytes, reducing preparation time and storage space required.
Analytes present at clinically relevant levels ensuring accurate test system performance across the clinical range, maximising laboratory efficiency by eliminating the need to purchase additional high or low-level controls at extra expense.
True third party controls designed to provide an unbiased assessment of performance, our Acusera controls have not been manufactured in line with or optimised for use with any particular reagent, method or instrument.
For more information on any of our products, or to request a consultation from one of our QC Consultants, contact us via acusera@randox.com.
What is Measurement of Uncertainty?
Measurement Uncertainty (MU) relates to the margin of doubt that exists for the result of any measurement, as well as how significant the doubt is. For example, a piece of string may measure 20 cm plus or minus 1 cm, at the 95% confidence level. As a result, this could be written: 20 cm ±1 cm, with a confidence of 95%. Therefore, we are 95% sure that the piece of string is between 19 cm and 21 cm long.
Standards such as ISO 15189 require that the laboratory must determine uncertainty for each test. However, they have not specified how this should be done.
How do we calculate Measurement Uncertainty using QC data?
Employing your QC data to calculate uncertainty makes several assumptions; your test system is under control, the patient samples are treated in the same manner as your controls and gross outliers have been removed. If you choose to use your QC data to calculate this you should ensure that you use a commutable control with a matrix similar to that of a patient sample, with analytes present at clinically relevant levels
To calculate MU, labs must look at the intra-assay precision and inter-assay precision of their test.
Intra-assay precision: Sometimes known as ‘within run’ precision, is where 20 or more replicates of the same sample are run at the same time, under the same conditions (calculated from a single experiment). Intra-assay precision helps to assess systematic uncertainties
Inter-assay precision: Sometimes known as ‘between run’ precision, is where 20 or more replicates are run at different times – e.g. 1 replicate every day for 20 days (can be calculated from routine IQC data). Inter-assay precision can help identify random uncertainties within the test system.
*The Australian Association of Clinical Biochemists (AACB) recommends that at least 6 months’ worth of QC data are used when calculating the inter-assay precision1.
Once the data is collected, you must calculate the standard error of the mean (SEM) of the intra-assay precision (A) and the SD of the inter-assay precision (B) in order to measure the uncertainty (u). Once A and B have been calculated, they need to be squared, added together and the square root of the sum found:
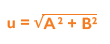
As uncertainty is calculated as SD and 1SD is equal to 68% confidence on a standard Gaussian curve, we can conclude that if we multiply using a coverage factor of 2, we can attain 2SD confidence of 95%. This is known as the Expanded Uncertainty (U):
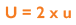
What is the Advantage of Measurement Uncertainty for a lab?
Labs need to carry out MU as it is a requirement of ISO 15189. It states: “The laboratory shall determine measurement uncertainty for each measurement procedure, in the examination phases used to report measured quantity values on patients’ samples. The laboratory shall define the performance requirements for the measurement uncertainty of each measurement procedure and regularly review estimates of measurement uncertainty”.
MU also helps determine whether the difference between two results is negligible due to uncertainty or significant due to a genuine change in condition of the patient; giving labs a greater confidence in reported results.
How can Randox help?
Our new Acusera 24.7 Live Online software provides automatic calculation of MU, saving valuable time and helping labs meet ISO 15189 requirements with ease.
Contact marketing@randox.com to find out how your lab can benefit from Acusera 24.7 Live Online
Fabricado en Europa – Randox Control de Calidad
Ubicados en Europa, la misión de Randox Control de Calidad es la producción de materiales de alta calidad que ayuden a racionalizar los procesos de control de calidad, además de ayudar a ahorrar tiempo y dinero a cualquier laboratorio, independientemente de su tamaño y presupuesto. Gracias a nuestra extensa oferta de productos, en los que se incluyen controles internos de tercera opinión, un software para la gestión de datos inter laboratorios y una amplísima variedad de Programas de Evaluación Externa de Calidad (PEEC), podrá obtener una visión completa del rendimiento de su laboratorio.
Al ser uno de los mayores fabricantes de controles auténticamente conmutables, ofrecemos soluciones para el control de calidad económicas, de la más alta calidad y diseñadas para la evaluación de los resultados de las pruebas de los pacientes. Con nuestra gama de controles Acusera podrá controlar lo que hasta ahora le supondría hasta 4 controles diferentes con un único vial Acusera, que junto con nuestra estabilidad sin igual y la elevada vida útil, le ayudarán a ahorrar tiempo y dinero. Además, las concentraciones son clínicamente relevantes, garantizando la precisión y fiabilidad de los instrumentos a lo largo de toda la variabilidad clínica.
Acusera 24.7 gestión de datos interlaboratorios
Acusera 24.7 le permitirá obtener resultados más precisos y reducir el tiempo que implica la supervisión e interpretación de los datos del control de calidad. Además, tendrá acceso a una gran variedad de funciones como a los datos del grupo par actualizados en tiempo real y el cálculo automático de la Incertidumbre, el Error Total y las Métricas Sigma. Con Acusera 24.7 podrá asegurar la calidad de sus análisis a través de la resolución más rápida de los problemas mediante esta plataforma centralizada.
RIQAS es el Programa de Evaluación Externa de Calidad (PEEC) de preferencia, empleado por más de 45.000 participantes en 124 países. Gracias a la frecuencia de los informes, podrá identificar los errores rápidamente, reduciendo la necesidad de realizar repeticiones innecesarias y costosas, haciendo que su laboratorio ahorre tiempo y recursos valiosos.
Take steps to prevent incorrect patient results by making one simple change
According to the NHS Litigation Authority; in 2015 within the UK alone, £193,680,744.30 was spent on ‘wrong diagnosis’ or ‘failed/delayed diagnosis’ causing huge financial strain and impact on labs.
With approximately 75% of clinical decisions and diagnosis based on laboratory test results. The only way to guarantee a high degree of accuracy is to implement a good Quality Control plan. The importance of this is recognised globally, several bodies exist internationally including ISO (International organisation for standardisation) who have developed a set of guidelines and quality systems to ensure the reliability of laboratory test results.
So what can you do to improve accuracy and reliability?
Choose a third party QC
ISO 151589:2012 Section 5.6.2.2 states that “the use of third party control materials should be considered, either instead of, or in addition to, any control materials supplied by the reagent or instrument manufacturer”.
First Party Controls are those manufactured by the instrument/reagent manufacturer. These controls are optimised specifically for use with the manufacturers test system and therefore will mask a multitude of weaknesses. First Party Controls tend to result in perceived accuracy and a biased assessment of performance.
Third Party Controls on the other hand are designed to be completely independent and are not optimised for use with a specific test or system. Leading manufacturers of third party controls will assign target values based on data collected from thousands of independent laboratories, ensuring the availability of statistically robust multi-method, multi-analyser data. Therefore laboratories using Third Party Controls can be assured of unbiased error detection across multiple platforms.
Randox Acusera is a world leading manufacturer of true third party controls providing a cost effective, high quality solution for any laboratory-regardless of size or budget.
Look out for QC samples with clinically relevant concentrations
ISO 15189:2012 states that ‘The laboratory should choose concentrations of control materials wherever possible, especially at or near clinical decision values, which ensure the validity of decisions made’.
It is important to assess the full clinical range of an assay i.e. the range between the lowest and highest results which can be reliably reported. In order to make sure a laboratory instrument is performing accurately across the full clinical range and in particular at the medical decision level, QC materials that cover low, normal and elevated concentrations should be used.
Due to the superior manufacturing process used by Randox, QC target values consistently cover the MDL of tests. By ensuring the controls in use cover clinical decision levels laboratories can be confident of the reliability and accuracy of the patient results they release.
Opt for a commutable control material
A good QC material has many essential properties but above all, controls must perform consistently and reflect the performance of patient samples – if a control meets these requirements then we can say it is commutable. Having a commutable control would aid in the prevention of incorrect patient results because they replicate the performance of a patient sample and react to the test system in a similar manner. Use of a commutable control will also reduce costly shifts in QC target values when reagent batch is changed.
At Randox we take quality seriously, that’s why all QC products are manufactured to the highest possible standard, delivering controls of unrivalled quality. Designed to be commutable, the Acusera range will ensure accurate and reliable instrument performance while simultaneously helping laboratories to meet ISO 15189:2012 requirements. A good QC process will include the use of Third Party Controls, Clinically Relevant Concentrations and controls which can be described as commutable. By employing Quality Control’s that encompass these traits, a laboratory professional can be certain that they have taken the necessary steps to decrease incorrect results and therefore potential misdiagnosis.
Acusera 24•7 Frequently Asked Questions (FAQ’s)

Is it possible to compare multiple instruments on a single Levey-Jennings Chart?
With Acusera 24•7 users can combine multiple instruments, as well as analytes and QC lots on a single chart. This will enable comparative performance assessment and facilitate immediate visualisation of any ongoing or emerging trends. It may also be useful for troubleshooting out of control QC events.
Is it possible to compare different levels of control on a single Levey-Jennings Chart for identification of concentration related bias?
Yes data for multiple QC lots can be displayed on a single, convenient Levey-Jennings chart allowing any concentration related problems to be identified quickly.
How often is peer group data updated?
With Acusera 24•7, peer group data is uniquely updated live in real-time. The instant nature of the peer data will help reduce time and money spent troubleshooting, re-running QC samples and performing any instrument maintenance. With real-time peer group data you can compare to other laboratories around the globe using the same lot of QC material and identify if there are any issues and whether they are unique to your lab or a widespread issue.
Is there a limit to the number of users?
There is no limit to the number of concurrent users – you can have as many as you want. There are 5 different levels of user access – Admin, Group Co-Ordinator, Manager, User and Technical Support. It is worth noting that User access can be customised per user to ensure access to only the required functionality
How can the software help me to meet ISO 15189:2012 requirements?
Unique to Acusera 24.7 , our software will automatically calculate Measurement Uncertainty (UM) and provide your laboratory with a printable report that can be used to help meet ISO 15189:2012 accreditation requirements. In addition to this the software can help to prevent the release of patient results in the event of a QC failure for example when the Quality Control rules are violated.
Is it possible to use RiliBAK as my allowable limits?
QC data can indeed be rejected or alerted based on RiliBAK guidelines. Other options are also available, including, CLIA, Biological Variation, RIQAS TDPA and user defined performance limits.
Is Acusera 24.7 secure?
To authenticate users, a number of security measures are used, including; participant number, username and a password combination (for individual role-based accounts). Password complexity standards are enforced on user account setup. CAPTCHA is enforced after several failed login attempts to prevent or guard against automated attacks. HTTPS and X509 certificate authentication is in place meeting industry security standards.
I have never used interlaboratory software before, is there training provided with my purchase of 247?
There are a number of different options available in terms of training. The easiest, most convenient and accessible form of training is through the use of our walk through demo that has voice-over and text so everyone can follow. With our software comes a user guide, this is a walk through that laboratories can use to guide them through the usage of the software. Additionally, there is also the possibility of a live demo from the sales consultant in your area who will be able to run through the software with you.
Can I create unique login credentials for each user?
Yes, each user will have their own personal log-in. If a lower level user, e.g. user, technical support or manager forgets their password they can have it reset by the administrator. However, if a group co-ordinator or admin forgets their password they should contact Randox directly who will be able to reset their credentials and resend to the administrator’s email address.
I currently use non-Randox Quality Controls, does this affect the ability of the software?
Not at all, our software is so flexible that it can be used with any other manufacturers Quality Control material. However, you will only have access to the internal functions of the software and will not have access to peer group statistics. For this reason we recommend the software is used alongside our Acusera true third party Quality Control solutions.
Is Acusera 24.7 the only software option available?
Yes this is the only option available – Unlike other manufacturers there is no need for any additional software packages or options. All functionality is available in the one software package.
My computer is very old, do I need a new, modern and up-to-date operating system to run the software?
Not necessarily. As long as you have stable access to the internet you can access Acusera 24.7 as it is a cloud based software.
My patient data is confidential. Will the software need access to this data?
Acusera 24.7 will not require access to your patient data. This is important for a laboratory but less for the software. Acusera 24.7 will only need access to the results of your Quality Control to ensure that your instrument/s are performing to standard and therefore ensuring that your patient results will be reliable and accurate.
I have forgotten my username and password – what do I do now?
If an individual with user level or manager level access forgets their username and password, they should contact the laboratory administrator. If an administrator or group co-ordinator forgets their username or password they should contact Randox who will verify the administrator and send new login details for the account.
Is Acusera 24.7 Connect required to import QC data?
Acusera 24.7 Connect is only required if you wish to import QC data automatically. Data can also be entered manually using the data entry screen or in a semi-automated manner using the EDI function.
I need to renew my license, is this done automatically?
If your licence has expired and you would like to renew you should get in touch with your local Sales Consultant.
My internet connection isn’t great. Will this affect the running of the software? What happens if the signal drops when entering results?
If connection is lost from the laboratory’s side, all data will be transferred to the web and once reconnected, the previous session will also be remembered. Emergency power generators and fall over servers are in place to ensure 99.8% uptime is guaranteed.
Are there any additional software requirements?
You must have access to a Java applet. This software is available as standard on almost all modern computers, laptops and notebooks.
Aliquoting for longer QC stability
Al-i-quot: An amount that is an exact divisor of the whole quantity of a substance (Collins Dictionary of Medicine, R. Young, 2005).
Why aliquot QC material?
Aliquoting QC material can extend the open vial stability of a lyophilised control, according to manufacturer recommendations. By splitting your QC material into a number of tubes and freezing these you can extend the working stability of the control, ultimately reducing wastage and the amount of money spent on unnecessary additional controls.
Example
A laboratory purchases a lyophilised QC with a volume of 3ml once reconstituted the control is stable for 7 days at 2-8oC. However, the laboratory only uses 1ml of this control per week, meaning that 2ml could potentially be wasted. The manufacturer states that the control can be frozen after reconstitution, extending the working stability from 7 days at 2-8oC to 30 days at -20 oC to -80oC. The following outlines the process for aliquoting reconstituted material and extending the control’s working stability.
Aliquoting reconstituted material
- Reconstitute the QC material according to the manufacturer’s instructions.
- Using a micropipette aliquot the required volume (generally a minimum of 0.5ml should be used) of reconstituted material into a tube.
- Repeat step 2 until all the reconstituted material has been aliquoted.
- Label each tube with the date the material was reconstituted to avoid the use of expired material.
- Store each aliquot at -20oC in a frost free freezer. Be sure to check the kit insert for frozen stability claims.
- Remove and thaw each aliquot as and when required making sure to use all material within the frozen stability period.
- Once thawed do not refreeze, dispose of any leftover QC material.
Conclusion
Aliquoting reconstituted material is an ideal way of extending the control’s open vial stability. This will ensure that your laboratory minimises the amount of QC material wasted and saves money by eliminating the need to purchase additional controls. Please note that not all lyophilised controls can be frozen like this. To ensure the controls you are selecting are suitable for aliquoting check the product’s kit insert or contact your supplier.
What can Randox Quality Control offer?
We have a number of lyophilised controls which can be prepared and stored in this way across our extensive product portfolio. To find out more visit www.randoxqc.com or contact us via acusera@randox.com to arrange a visit from one of our QC Consultants.
The Benefits of Peer Group Data to your Troubleshooting Process
Drive for more accurate results in your laboratory
We’ve all been there, you’re in the middle of a run of patient tests when you are alerted to an out of control event, such as your analyser is reporting QC results 25% low to target. What do you do? In reality, we all know that the problem is unlikely to correct itself, especially if it’s a calibration or analyser issue. Human error is a potential factor, however all possible causes must be eliminated to proceed with patient testing.
What’s the solution?
ISO 15189:2012 recommends that a laboratory should “have a procedure to prevent patient results in the event of a quality control failure”. Implementing an interlaboratory data management program which features peer group reporting can help you meet this requirement and monitor the results you are producing. Such programs can help detect errors in the analytical phase of patient testing, through the automatic application of pre-programmed QC rules, thus alerting staff to failed results.
Why must Peer Groups be a feature?
A peer group is defined as a “Community in which most or all members have roughly the same characteristics…” (Businessdictionary.com, accessed 2017). In this instance the characteristics could refer to the; instrument, test method or QC material in use. As such peer group programmes could help you detect errors in your laboratory by comparing your results to those who are employing a similar method, instrument and QC to what you are using, i.e. comparing apples for apples. Therefore it is essential that the peer group data you require is available in real-time, to ensure you are accessing the most up-to-date data when reviewing your patient test results.
Scenario
Take the example from the introduction. You’re in the middle of a run of patient tests when you are alerted to an out of control event, such as your analyser is reporting QC results 25% low to target. As part of your troubleshooting procedures, you are able to compare your results to the results of your peer group and note that this is an isolated incident. Consequently, you have eliminated a widespread problem with the QC, reagent or calibrator and narrowed down the root cause to one of the components in your test system. Thus saving you time in the troubleshooting process.
Benefits of Peer Group Comparison
There are a number of benefits to employing peer group comparison in your laboratory. Peer group data comparisons facilitate faster troubleshooting, helping you identify whether the problem you are seeing is unique to your laboratory, or if other laboratories are reporting the same issue. If other laboratories are reporting the same issue it is possible to conclude that there is a widespread problem with either the QC, reagent or calibrator. On the other hand, if it is not occurring within your peer group you will have to investigate further, reviewing your QC processes. As a result, you could resolve issues much quicker by eliminating either a supplier or laboratory issue. Furthermore, you can also eliminate the need for unnecessary repeat tests or instrument maintenance, saving both valuable time and money.
Other characteristics you should look out for
Whilst peer group comparison is a useful feature there are a number of other features you should consider when selecting the right interlaboratory data management program for you. These include;
- Automatic calculation of Measurement Uncertainty, Total Error and Sigma Metrics
- Multiple laboratory management on a single platform
- Accessing data anytime, anywhere via PC, laptop or tablet via a web-based platform
- All data charts you may require to assess whether any bias or imprecision issues are present
- Ability to combine data for multiple QC lots, analytes and instruments on a single Levey-Jennings or Histogram chart
- Automated data import via a direct connection to your LIMS
What can Randox offer?
At Randox we are passionate about quality control and believe in producing high-quality material that can streamline procedures for laboratories of all sizes and budgets through our Randox Quality Control brand. Acusera 24.7 Live Online is just one aspect of our extensive laboratory portfolio that has been designed to help you produce results you can trust. With Acusera 24.7 Live Online you can drive for more accurate results by monitoring and interpreting QC data online, anytime, anywhere. With access to an impressive range of features, including the automatic calculation of Measurement Uncertainty, Total Error and Sigma Metrics, Acusera 24.7 will ensure analytical quality.









