Acusera Apolipoproteins Linearity Verifier

Dedicated Linearity Verifier for measuring Apo A-1 and Apo B on Roche Cobas analysers. Supplied in a liquid frozen format this linearity verifier will objectively verify calibration of the
instrument whilst remaining convenient and easy to use. Five levels are provided spanning the instrument’s reportable range.
Features & Benefits
- Convenient, liquid frozen format
- 5 levels provided
- 14 day stability when stored at 2ºC to 8ºC
- Shelf life of up to 2 years from date of manufacture
| Description | Size | Analytes | Cat No | |
|---|---|---|---|---|
| Acusera Apolipoproteins Linearity Verifier | 5 x 3mL | 2 | LV10363 | |
Analytes
- Apolipoprotein A-1 (Apo A-1)
- Apolipoprotein B (Apo B)
Acusera Lipids Linearity Verifier



Our Lipids Linearity Verifier comprises 4 common lipid assays and is specifically designed for use on Roche Cobas analysers. Five levels are available and span the instrument’s complete reportable range. Designed in a liquid frozen format, this linearity verifier will objectively verify calibration of the instrument whilst remaining convenient and easy to use.
Features & Benefits
- Convenient, liquid frozen format
- 5 levels provided
- 14 day stability when stored at 2ºC to 8ºC
- Shelf life of up to 2 years from date of manufacture
| Description | Size | Analytes | Cat No | |
|---|---|---|---|---|
| Acusera Lipids Linearity Verifier | 5 x 3mL | 3 | LV10364 | |
Analytes
- HDL Cholesterol
- LDL Cholesterol
- Total Cholesterol
- Triglycerides
Acusera RF Linearity Verifier




Dedicated Rheumatoid Factor (RF) Linearity Verifier supplied in a liquid ready-to-use format specifically for use on Roche Cobas analysers. Designed to objectively verify calibration
whilst remaining convenient and easy to use, there are five distinct levels provided that span the instrument’s complete reportable range.
Features & Benefits
- Convenient, liquid ready-to-use format
- 5 levels provided
- 14 day stability when stored at 2ºC to 8ºC
- Shelf life of up to 2 years from date of manufacture
| Description | Size | Analytes | Cat No | |
|---|---|---|---|---|
| Acusera RF Linearity Verifier | 5 x 1mL | 1 | LV10343 | |
Analytes
- Rheumatoid Factor (RF)
Acusera Esoterics Linearity Verifier




Our Esoterics Linearity Verifier comprises 6 analytes and is supplied in a liquid ready-to-use format specifically for use on Roche Cobas analysers. Designed to objectively verify calibration whilst remaining convenient and easy to use, there are five distinct levels provided that span the instrument’s complete reportable range.
Features & Benefits
- Convenient, liquid ready-to-use format
- 5 levels provided
- 14 day stability when stored at 2ºC to 8ºC
- Shelf life of up to 2 years from date of manufacture
| Description | Size | Analytes | Cat No | |
|---|---|---|---|---|
| Acusera Esoterics Linearity Verifier | 5 x 3mL | 6 | LV10336 | |
Analytes
- Acetaminophen
- Ammonia
- Ethanol
- Microalbumin
- Urinary Protein
- Salicylate
Acusera hsCRP Linearity Verifier




Dedicated hsCRP Linearity Verifier supplied in a liquid ready-to-use format specifically for use on Roche Cobas analysers. Designed to objectively verify calibration whilst remaining convenient and easy to use, there are five distinct levels provided that span the instrument’s complete reportable range.
Features & Benefits
- Convenient, liquid ready-to-use format
- 5 levels provided
- 14 day stability when stored at 2ºC to 8ºC
- Shelf life of up to 2 years from date of manufacture
| Description | Size | Analytes | Cat No | |
|---|---|---|---|---|
| Acusera hsCRP Linearity Verifier | 5 x 1mL | 1 | LV10335 | |
Analytes
- High Sensitivity C-Reactive Protein (hsCRP)
Acusera CRP Linearity Verifier




This dedicated CRP Linearity Verifier is supplied a liquid ready to-use format, specifically for use on Roche Cobas analysers. This verifier is designed to objectively verify calibration whilst remaining convenient and easy to use. There are five distinct levels provided that span the instruments’ complete reportable range.
Features & Benefits
- Convenient, liquid ready-to-use format
- 5 levels provided
- 14 day stability when stored at 2ºC to 8ºC
- Shelf life of up to 2 years from date of manufacture
| Description | Size | Analytes | Cat No | |
|---|---|---|---|---|
| Acusera C-Reactive Protein (CRP) Linearity Verifier | 5 x 1mL | 1 | LV10334 | |
Analytes
- C-Reactive Protein (CRP)
Product Spotlight: HbA1c Quality Controls
Diabetes
Diabetes is a life-long condition which occurs when the glucose level in the blood is too high because it can’t enter the body’s cells to be used as fuel. There are two types of diabetes: type 1 and type 2. They are distinct conditions and must be treated and managed differently.
Type 1 Diabetes
Type one diabetes is an autoimmune condition in which the body attacks insulin-producing cells, this causes a lack of insulin, leading to an increased blood glucose level. Around 10% of people with diabetes has type 1.
Type 2 Diabetes
A mixture of genetic and environmental factors causes type 2 diabetes. The body doesn’t make enough insulin or the insulin it does create does not work correctly, leading to a glucose build up in the blood. It’s thought that up to 58% of type 2 diabetes can be prevented or delayed through healthy lifestyle choices.
HbA1c
HbA1c is the average blood glucose level for the past two to tthree months. A high HbA1c means there is too much sugar in the bloodstream. This means the patient is more likely to develop complications associated with diabetes, like problems with feet and eyes [1].
HbA1c in Diagnostics
In 2011, the WHO accepted the use of glycated haemoglobin (HbA1c) testing in the diagnosis of diabetes, Diabetes UK also supports this decsion [2].
Clinically Significant Levels
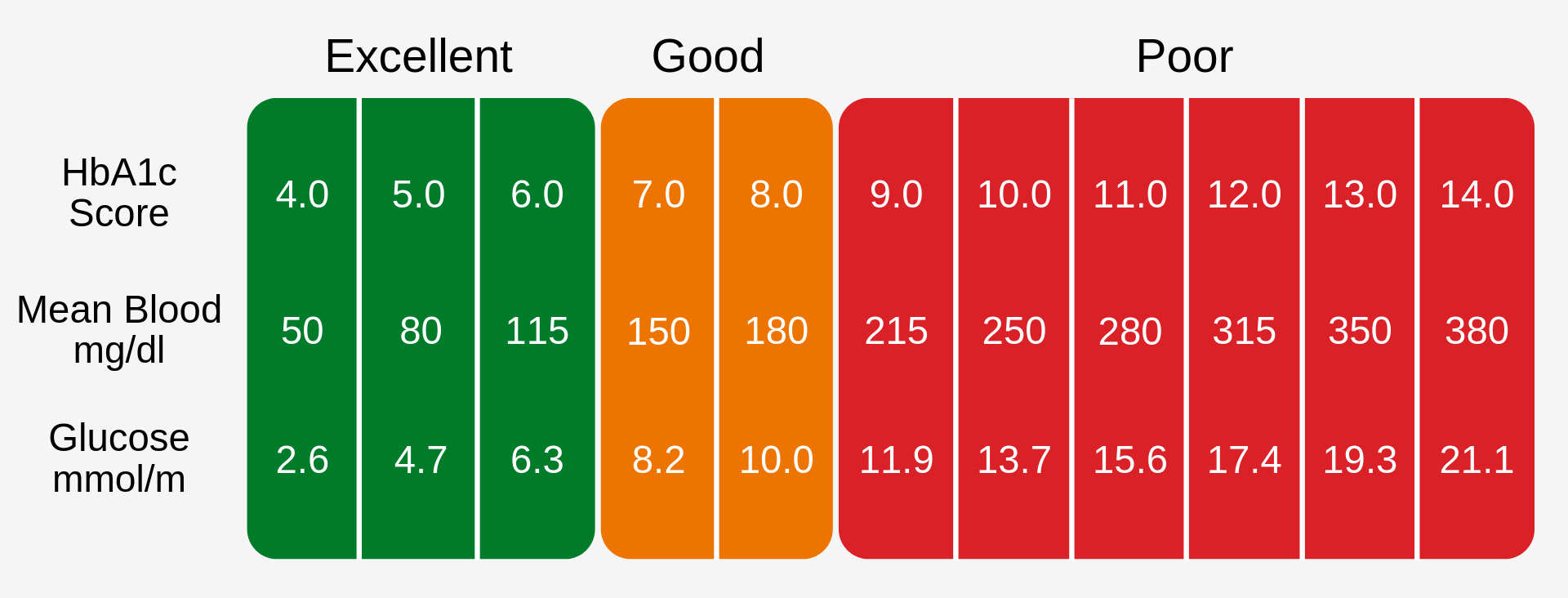
A HbA1c level of 6.5% is recomended as the cut off for diabetes diagnosis [3], this can be seen in Fig. 1.
Acusera HbA1c Controls
Acusera HbA1c Quality Control
The Randox Acusera HbA1c control is designed for use in the quality control of both HbA1c and Total Haemoglobin assays. Assayed instrument and method specific target values and ranges are provided for all major systems and methods including HPLC. A reconstituted stability of 4 weeks keeps waste to a minimum and helps to reduce costs.
Acusera Liquid HbA1c Quality Control
Conveniently supplied liquid ready-to-use the Liquid HbA1c control is ideally suited to both clinical laboratories and POCT helping to significantly reduce preparation time. With a stability of 30 days waste and costs are also kept to a minimum.
Flu Season
Molecular Respiratory Testing
Contact Us
Product Spotlight Home
Visit the Product spotlight Home to see past product spotlights
References
[1] “What is HbA1c?”, Diabetes UK, 2018. [Online] Availabel: https://www.diabetes.org.uk/guide-to-diabetes/managing-you-diabetes/hba1c.
[2] Diabetes UK, “Diagnostics criteria for deabetes”, Diabetes UK, 2018. [Online]. Available: https://www.diabetes.org.uk/professionals/position-statements-reports/diagnosis-ongoing-management-monitoring/new_diagnostic_criteria_for_diabetes.
[3] WHO, “Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus”, World Health Organisation, 2011.
sTfR Quality Control




Providing a true third party solution for the measurement of Soluble Transferrin Receptor (sTfR), the Acusera sTfR Control will deliver an unbiased, independent assessment of analytical performance.
Designed for use with sTfR assays, this handy single analyte control saves money on wasted material.
Features & Benefits
- Lyophilised control
- Human based material
- Assayed target values available
- Stable to expiry date at 2°C to 8°C
- Reconstituted stability of 30 days at 2°C to 8°C
| Description | Size | Analytes | Cat No | |
|---|---|---|---|---|
| sTfR Control (Bi-level) | 3 x 2 x 1 ml | 1 | TF10162 | |
| sTfR Calibrator | 6 x 1 ml | 1 | TF10161 | |
Analytes
- Soluble Transferrin Receptor (sTfR)
Related Products
Metabolic Syndrome Array II Quality Control




A multi-analyte quality control with target values and ranges provided for 3 parameters associated with metabolic syndrome.
Features & Benefits
- Lyophilised for enhanced stability
- 100% human material
- Stable to expiry date at 2oC – 8oC
- Reconstituted stability of 72 hours at 2oC – 8oC and 7 days at -20°C
- Assayed values available for Randox Biochip systems
| Description | Size | Analytes | Cat No | |
|---|---|---|---|---|
| Metabolic Syndrome Array II Control | 3 x 3 x 1ml | 3 | EV3761 | |
| Metabolic Syndrome Array II Calibrator | 9 x 1 ml | 3 | EV3760 |
Analytes
- Adiponectin
- CRP
- Cystatin C