RIQAS Point of Care EQA
RIQAS Point of Care EQA
Designed to improve the quality of Point of Care Testing (POCT) in locations such as pharmacies, GP surgeries, hospital out patient departments, sports clinics, supermarkets, diagnostic/treatment and walk-in centres.
RIQAS Point of Care EQA provides independent evidence of the accuracy and reliability of test results.
Why RIQAS Point of Care?
Programme Offering
Tests
Role
Matrix
Lipids (Total Cholesterol & HDL Cholesterol)*
- Risk Factor for heart Disease
- Monitoring lipid lowering therapy
Whole Blood
HbA1c (Glycated Hemoglobin)*
- Diagnosing diabetes mellitus
- Monitoring treatment
- Encouraging self-management
Whole Blood
CRP (C Reactive Protein)*
- Early detection of infectious disease
- Identifying need for antibiotic treatment
Whole Blood
Glucose / Ketones
- Diagnose and monitor diabetes
- Monitor the presence of hypoglycemia (low blood glucose) and hyperglycemia (high blood glucose)
- To determine whether excessive ketones are present in the blood, to detect diabetic ketoacidosis (DKA)
Serum
International Normalised Ratio (INR)
- Used to measure the effect of anticoagulant drugs such as warfarin
- Help diagnose a bleeding disorder; to help estimate the severity of liver disease
Whole Blood
Key Cycle Dates
RQ9181 Distribution Month Sample Distributed Result Submission Deadline January 2024 8th January 17th January February 2024 5th February 14th February March 2024 4th March 13th March April 2024 2nd April 10th April May 2024 6th May 15th May June 2024 3rd June 12th June July 2024 1st July 10th July August 2024 5th August 14th August September 2024 2nd September 11th September October 2024 7th October 16th October November 2024 4th November 13th November December 2024 2nd December 11th December What Participants Say
What participants say
Our unrivalled commitment to quality and service ensures high levels of customer satisfaction, this is evident from the responses to our latest customer satisfaction survey:
“All in all a quick and efficient service”
“Good online system”
“Very helpful team”
“Excellent training”
“They are an experienced team”
“Very satisfied with the service that we receive”
“Very good value for money”
“The website is great”
The Importance of Quality Assurance
Importance of quality assurance
Quality assurance is an essential aspect of any clinical/diagnostic testing service and is aimed at ensuring the accuracy and reliability of patients’ results. The right result allows the right clinical advice to be offered in a timely manner. Quality assurance operates at two levels:Internal Quality Control
Internal Quality Control includes operator training/ competency assessment, analyser/ test system maintenance, and adherence to policies/ processes. Whilst some point of care analysers include inbuilt quality checks, cross-check analysis against samples with known levels provides immediate assurance and evidence that a patient’s result is safe to report.External Quality Assessment
External Quality Assessment involves analysis of samples with unknown levels that have been distributed by an external organisation. Participants are informed how their results compare with other participants, hence providing independent evidence of performance. Increasingly, participation in an external quality assessment scheme is becoming a mandatory requirement where health and healthcare services are being provided.
EQA provides assurance to both staff and customers that testing provides accurate and reliable results.
Want to know more?
Contact us or download the RIQAS Point of Care catalogue to learn more.
Continue Reading
Frequently Asked Questions
RIQAS
Contact Us
Welcome to Vivasuite!

Running within the Bosch IoT cloud maintaining the highest standard of IT security and data privacy. Connectivity ensures that devices are always available and fully updated.
Advantages of Vivasuite:
– Schedule remote software updates
– Know when your devices were last synchronised
– View all devices in one dashboard
– View device information and general device data
– View testing history from one system
– Mobile device monitoring
– Vivalytic user management
– Save time with less “on-device” work
Vivalytic Overview
Discover more about the Vivalytic
VeraSTAT-Sports Performance
Best in the Field
![Running Man Vector Green [Converted]](https://www.randox.com/wp-content/uploads/2023/02/Running-Man-Vector-Green-Converted-1024x707.png)
![Running Man Vector Green [Converted]](https://www.randox.com/wp-content/uploads/2023/02/Running-Man-Vector-Green-Converted-1024x707.png)
VeraSTAT-Improve your performance
As the best it’s field, the Randox VeraSTAT device allows athletes to overcome the limitations of other generation tests, providing accurate, cost effective and reliable results that will help users receive the care necessary to get them back on their feet and back to their best.
Monitoring response to exercise is vitally important to an athlete and trainer. While a heavy training schedule can lead to chronic immunosuppression in athletes, it is essential that they receive the appropriate care in the case of a dip in health state. Eliminating the risk of inflammation and infection is essential to preventing disruptions to practice and performance.
VeraSTAT Test Menu
The VeraSTAT testing menu is designed to monitor an athletes immune response to exercise. C-Reactive Protein (CRP) levels are used to guide the treatment of bacterial infections or inflammation associated with tissue injury and other inflammatory disorders. On the other hand, Mxyovirus Resistance Protein 1 (MxA) is used as a key indicator of viral infections. These tests used in combination, can allow healthcare clinicians to determine the best course of treatment and get the athlete back to full health
C-Reactive Protein (CRP)
CRP is a Key indicator of inflammation and stress, often resulting from the breakdown in tissues. Overtraining can lead to elevated levels of CRP in the body.
Myxovirus Resistance Protein I (MxA)
MxA is a key indicator of a viral infection which may impact physical performance and activity levels. Unexplained failures are often attributed to recent or current infections.
Vivalytic Resource Hub
Rapid PCR MRSA/SA testing now available on Vivalytic
Rapid PCR MRSA/SA testing now available on Vivalytic
Providing a quick diagnosis of methicillin resistant at the point of the care, the latest addition to the Vivalytic portfolio of tests, not only provides rapid RT-PCR results in 53 minutes but differentiates whether the bacterial strain is methicillin-resistant (MRSA) or methicillin-sensitive (MSAA) which promotes targeted therapy.
MRSA is a major multi-resistant nosocomial pathogen worldwide with the WHO estimating that the mortality rate of patient infection rates is around 50% higher compared with patients who have been infected by non-resistant Staphylococcus aureus strains.1 Moreover, the extensive period of hospitalisation, morbidity, and the associated medical costs increase significantly with an MRSA infection.2
Introducing MRSA to the vivalytic portfolio can provide high quality answers, anywhere and anytime improving patient pathways and the need for care. Significantly, introducing rapid MRSA screening at both ward level, emergency settings and before hospital elective surgery procedures allow for an effective response to identifying whether the bacteria strain is methicillin-sensitive (MSSA) or -resistant.
Making a point to care, the rapid essence and speed of Vivalytic not only showcase technology but the ability to contribute to current health risks by preventing contamination, breaking the chain of infection, and again fighting the silent pandemic of antimicrobial resistance (AMR) & superbugs.
The treatment on the front line today looks at increasing empirical antibiotic prescribing and increasing drug-resistant outbreaks. AMR is growing rapidly, with superbugs threatening the ability to treat common infectious diseases appropriately. The COVID-19 pandemic has elevated concerns over AMR and antibiotic-associated adverse events, with surges in antibiotic prescribing, hospitalisations, and drug-resistant bacterial transmissions.
Speed is key here – since the result of diagnostics with culture sampling, which is the current traditional method for MRSA testing is only available after one to three days, this PCR test for the point of care is ideal as an additional tool when speed is of the essence.
Few points to note about the current Vivalytic panel for MRSA/SA detection:
- By using one single cartridge, the Vivalytic MRSA/SA test detects and differentiates between MRSA and MSSA DNA to aid in the diagnosis of MRSA infection in a speedy manner so that appropriate antibiotic treatment can be applied, and complications prevented.
- Detection Method: Real-Time PCR
- Result Time: 53 minutes
- Sample Volume: 600 μl
- Sample Type: Nasal- or oropharyngeal swab sample


| DETECTABLE DNA PATHOGENS: | SPECIFIC GENE TARGETS: |
|---|---|
| Methicillin-resistant Staphylococcus aureus (MRSA) | SCCmec/orfX junction |
| Methicillin-sensitive Staphylococcus aureus (MSSA) | mecA/ mecC, SA422 |
Making this happen, The MRSA/SA rapid test on Vivalytic by Bosch, a point of care platform brought to the market by Randox Laboratories. The Vivalytic system is a fully automated, cartridge-based platform capable of both Hi-Plex and Lo-Plex infectious disease testing. Each easy-to-use cartridge contains all necessary reagents, is fully-sealed to minimise risk and can be conveniently stored at room temperature.
The Vivalytic consolidates the full molecular workflow into a small benchtop platform, capable of extraction, PCR amplification and detection. It follows an easy 4 step process from sample entry to results and with the gold standard PCR testing. With most up to date technology, the Vivalytic has wireless connectivity, with no peripherals required, making a unique space saving and hygienic solution. Handling and utilisation are simple and medical professionals require only minimal training.


For more information on the Vivalytic, why not visit our webpage- https://www.randox.com/vivalytic-molecular-point-of-care/
For more information on our new MRSA test, please contact market@randox.com
ABOUT RANDOX
NEWS
VACANCIES
OUR PEOPLE
Identification and Differentiation of Viral and Bacterial Respiratory Infection to Guide Antibiotic Stewardship


Identification and Differentiation of Viral and Bacterial Respiratory Infection to Guide Antibiotic Stewardship
The development of point-of-care testing is critical in the identification and differentiation between bacterial and viral respiratory infections. Defining the indications of infection to improve antibiotic stewardship, ensures that patients are protected from unnecessary antibiotic use and antibiotic resistance. It has been shown that particular protein biomarkers, such as myxovirus resistance protein (MxA) and C-reactive protein (CRP), differentiate infections between bacterial and viral. Using point-of-care platforms, such as Randox’s VeraSTAT, for detection of these protein biomarkers may provide more rapid and cost-effective discriminating tools.
The treatment of bacterial and viral infections can differ significantly, however people are often treated with empirical antibiotics due to a lack of paid and accurate testing. Although early intervention of infection is urgent, current diagnostic methods are either time intensive or inaccurate. The challenges clinicians are faced with in the differentiation of viral or bacterial respiratory infection can lead to delayed diagnosis, misappropriation of antibiotics and increased healthcare costs.
MxA protein has the potential to greatly enhance the rapid detection of viral respiratory infections as it increases significantly when there is actuate viral infection. CRP is the dominant acute phase protein often used to guide treatment of a bacterial infection or inflammation associated with tissue injury, inflammatory disorders, and associated diseases.


CRP & MxA together, allow clinicians to make appropriate decisions in supporting antimicrobial stewardship and guide the appropriate use of antibiotics, saving time performing unnecessary tests, providing unnecessary treatment which missing the opportunity to provide the right treatment in a timely manner.
The Randox VeraSTAT is a simple, accurate, portable point of care device which delivers rapid results via the use of patented cathodic electrochemiluminescence technology (C-ECL). Designed with the aim of offering users the next generation of rapid diagnosis, the VeraSTAT eliminates the requirement to send samples to a laboratory and instead returns results in as little as 6 minutes.
- Eliminates delays in sending samples to the lab and facilitate immediate decision making at the point of care.
- Lightweight, portable and convenient, the Randox VeraSTAT can be used in a variety of locations to deliver results as required, such as a GP surgery or Emergency Department.
- Intuitive user interface guides the operator through the entire testing process.
- All necessary reagents are conveniently included in each single use, sealed cassette with no preparation required. All necessary consumables are supplied with the kit.
- The Randox VeraSTAT allows for results to be exported via Bluetooth connectivity.
- Flexible test menu comprising of a range of immunoassay, protein, inflammatory, diabetes & infectious disease markers.
Novel testing approaches identifying the type of infection at the point of care are essential in accurately guiding appropriate antibiotic treatment. Although these tests can’t determine what type of viral or bacterial infection a patient has, it will determine whether the infection is viral or bacterial, further testing is then carried out to determine what type of pathogen the patient has via PCR – the gold standard. The ability to distinguish between viral and bacterial infections is the most effective guide for clinical decision making and is an innovative tool for antibiotic stewardship.
References
1 – Fleming-Dutra K.E., Hersh A.L., Shapiro D.J. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA. 2016;315:1864–1873. doi: 10.1001/jama.2016.4151.
2 – Cals JW, Hopstaken RM, Butler CC, Hood K, Severens JL, Dinant GJ. Improving management of patients with acute cough by C-reactive protein point of care testing and communication training (IMPAC3T): study protocol of a cluster randomised controlled trial. BMC Fam Pract. 2007;8:15.
3- New report calls for urgent action to avert antimicrobial resistance crisis [Internet]. World Health Organization. World Health Organization; 2019
4 – Hutchings MI, Truman AW, Wilkinson B. Antibiotics: past, present and future. Curr Opin Microbiol. (2019) 51:72–80. doi: 10.1016/j.mib.2019.10.008
For more enquiries please contact the Marketing team: market@randox.com
ABOUT RANDOX
NEWS
VACANCIES
OUR PEOPLE
CRP & MxA VeraSTAT
VeraSTAT | CRP & MxA
Rapid Differentiation of Viral & Bacterial Respiratory Infections
MxA protein has the potential to greatly enhance the rapid detection of viral respiratory infections and increases significantly when there is actuate viral infection.
CRP is the dominant acute phase protein often used to guide treatment of a bacterial infection or inflammation associated with tissue injury, inflammatory disorders, and associated diseases.
Together, allow clinicians to make appropriate decision in supporting antimicrobial stewardship and guide thappropriate use of antibiotics.
MxA
VeraSTAT MxA kit is an in vitro near-patient diagnostic test for the quantitative determination of Myxovirus resistance protein A (MxA) from whole blood. The MxA Kit is used for detection of acute respiratory tract viral infections from symptomatic patients.
CRP
VeraSTAT CRP kit is an in vitro near-patient diagnostic test for the quantitative determination of C-reactive protein (CRP) from whole blood to assess the inflammatory status of the body.
Sample Volume- 7 μL
Sample Type- Whole Blood
Measuring Time- 11 minutes
Ordering Information: VS1004
Sample Volume- 5 μL
Sample Type- Whole Blood
Measuring Time- 6 minutes
Ordering Information: VS1003
Useful Resources
VERASTAT-V
GET IN TOUCH
VERASTAT BROCHURE
VeraSTAT
VeraSTAT | Excellence at the Point of Care
Accurate & Reliable Results in Minutes
Why Choose the VeraSTAT?
The Randox VeraSTAT is a simple, accurate, portable point of care device which delivers rapid results via the use of patented cathodic electrochemiluminescence technology (C-ECL).
Through this technology, the target analyte in the patient sample reacts with the labelled antibody and captured antibody. After the reaction, unbound or excess labelled antibody is washed away and the labelled antibody complex is excited with electricity, with the electrochemiuminescence being measured and an accurate result produced.
Designed with the aim of offering users the next generation of rapid diagnosis, the VeraSTAT eliminates the requirement to send samples to a laboratory and instead returns results in as little as 6 minutes when and where required.
The superiority of the VeraSTAT allows for users to overcome performance limitations of previous generation tests relating to sensitivity, accuracy, ease of use and cost efficiency. This, combined with a versatile test menu, means that the Randox VeraSTAT is built to outshine and enhance detection in any setting.
Click here to download Windows App:
- Download VeraSTAT Analyzer windows application to your computer.
- Extract the zipped file and double click VeraSTATsetup
- Follow the application install instruction to install the application on device
VeraSTAT User Guide
VeraSTAT Test Menu
C-Reactive Protein (CRP)
CRP is the dominant acute phase protein often used to guide treatment of a bacterial infection or inflammation associated with tissue injury, inflammatory disorders and associated diseases.
Myxovirus Resistance Protein I (MxA)
An informative general marker for the most common acute viral infections. MxA protein has the potential to greatly enhance the rapid distinction between viral and bacterial respiratory infections.
Useful Resources
VERASTAT-V
GET IN TOUCH
VERASTAT BROCHURE
RIQAS Point of Care EQA
Designed to improve the quality of Point of Care Testing (POCT) in locations such as pharmacies, GP surgeries, hospital out patient departments, sports clinics, supermarkets, diagnostic/treatment and walk-in centres, RIQAS Point of Care EQA provides independent evidence of the accuracy and reliability of test results. Randox International Quality Assessment Scheme (RIQAS) is the world’s largest EQA scheme with over 55,000 participants in more than 134 countries.
Why RIQAS Point of Care?
About RIQAS Point of Care
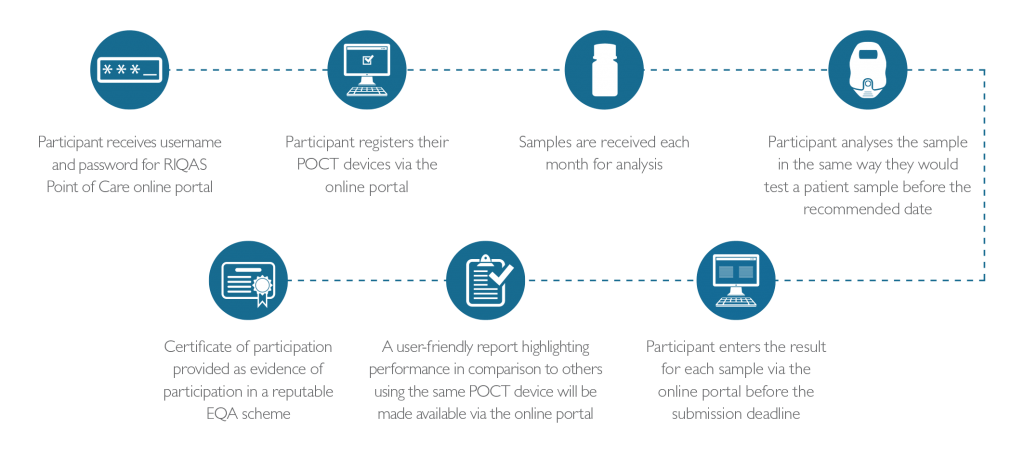
How it Works
Tests and Analysers
Test Role Matrix Lipids (Total Cholesterol & HDL Cholesterol) • Risk factors for heart disease
• Monitoring lipid lowering therapy
Whole Blood HbA1c (Glycated Haemoglobin) • Diagnosing diabetes mellitus
• Monitoring treatment
• Encouraging self-management
Whole Blood C-Reactive Protein (CRP) • Early detection of infectious disease
• Identifying need for antibiotic treatment
Whole Blood Glucose/Ketones • Diagnose and monitor diabetes
• Monitor for the presence of hypoglycaemia
(low blood glucose) and hyperglycaemia (high blood glucose)
• To determine whether excessive ketones are present in the blood, to detect diabetic ketoacidosis (DKA)
Serum International Normalised Ratio (INR) • Used to measure the effect of anticoagulant
drugs such as warfarin
• Help diagnose a bleeding disorder; to help
estimate the severity of liver disease
Plasma Note – The RIQAS Point of Care range is constantly expanding to include new tests and analysers. Please contact us if your desired analyser or test is not currently displayed.Ordering Information
Panel Catalogue Number Lipids RQ9181/A Lipids + 1 panel RQ9181/B Lipids + 2 panels RQ9181/C Additional Sample RQ9181/D Glucose and Ketones RQ9188 INR RQ9189 Pipette Tips RQ9182 Bulbous pipette RQ9183 What Participants Say
Our unrivalled commitment to quality and service ensures high levels of customer satisfaction, this is evident from the responses to our latest customer satisfaction survey:
“All in all a quick and efficient service”
“Good online system”
“Very helpful team”
“Excellent training”
“They are an experienced team”
“Very satisfied with the service that we receive”
“Very good value for money”
“The website is great”Key Cycle Dates
RQ9181 Distribution Month Sample Distributed Result Submission Deadline January 2024 8th January 17th January February 2024 5th February 14th February March 2024 4th March 13th March April 2024 2nd April 10th April May 2024 6th May 15th May June 2024 3rd June 12th June July 2024 1st July 10th July August 2024 5th August 14th August September 2024 2nd September 11th September October 2024 7th October 16th October November 2024 4th November 13th November December 2024 2nd December 11th December Importance of Quality Assurance
Quality assurance is an essential aspect of any clinical/diagnostic testing service and is aimed at ensuring the accuracy and reliability of patients’ results. The right result allows the right clinical advice to be offered in a timely manner. Quality assurance operates at two levels:Internal Quality Control
Internal Quality Control includes operator training/ competency assessment, analyser/ test system maintenance, and adherence to policies/ processes. Whilst some point of care analysers include inbuilt quality checks, cross-check analysis against samples with known levels provides immediate assurance and evidence that a patient’s result is safe to report.External Quality Assessment
External Quality Assessment involves analysis of samples with unknown levels that have been distributed by an external organisation. Participants are informed how their results compare with other participants, hence providing independent evidence of performance. Increasingly, participation in an external quality assessment scheme is becoming a mandatory requirement where health and healthcare services are being provided.
EQA provides assurance to both staff and customers that testing provides accurate and reliable results.Want to know more?Visit our Importance of EQA page to learn more.
Want to know more?
Contact us or download the RIQAS Point of Care catalogue to learn more.
Continue Reading
Frequently Asked Questions
RIQAS
Contact Us
Randox Sepsis innovation hailed by Health Secretary Matt Hancock
An innovative new tool for quickly diagnosing the often deadly infection Sepsis, will save lives, the Health Secretary has said.
The bedside test, being developed by healthcare diagnostics company Randox, will slash the 24 hours usually taken to identify the correct antibiotic for sepsis treatment. Currently, more than a third of those with sepsis die. Every hour that patients are not diagnosed increases the chance of death by 8%.
Health Secretary Matt Hancock said: “Instead of having to give people huge amounts of antibiotics across the board, which causes other problems, both medical and problems with resistance and super bugs, instead we will be able to work out exactly what the right treatment is for that individual person and do it fast enough to get the treatment in to save lives.”
He paid a visit to Randox’s new headquarters, the Randox Science Park, in Antrim, Northern Ireland on Thursday 21st March.
He added: “I can see a very clear application across the health service for how we can use the technology that is being developed here in Northern Ireland, both across the UK and indeed around the world.”
Sepsis can develop from infections caused by a simple cut or minor medical procedure. The body’s white blood cells fight the infection but the reaction can escalate and also damage healthy tissue.
Many who survive face amputations because of this tissue damage, Randox’s Molecular Diagnostics Manager Dr Martin Crockard said.
Dr Crockard highlighted that the traditional sepsis testing method, which involves sending blood samples to laboratories, takes too long. The problem is worsened by the fact that doctors are then forced to initially prescribe broad spectrum antibiotics which are not specific enough for individual patients. This encourages resistant strains.
To speed up the process, the new technology from Randox’s Biosciences division will allow clinicians in hospital emergency departments to check multiple samples simultaneously, at the press of a few buttons on a smart pad.
Dr Crockard said it is imperative that appropriate antibiotic treatment is administered as quickly as possible.
He said: “We can deal with the exact organism causing the problem in less than four hours, allowing you to tailor the treatment for that individual patient very quickly.”
The UK Sepsis Trust’s Chief Executive Ron Daniels said: “Randox is leading the way around molecular technologies.
“No other system brings this so close to the clinician on the shop floor.”
For further information please contact the Randox PR team by emailing randoxpr@randox.com or phoning 028 9442 2413