Importance of External Quality Assessment (EQA)

External Quality Assessment (EQA) / Proficiency Testing (PT) allows for a comparison of a laboratory’s testing procedures to other laboratories across the world. Comparisons can be made to a peer group of laboratories or to a reference laboratory.
EQA involves running blind patient-like samples, comparing your results to peer results, in order to retrospectively monitor the accuracy of reporting. EQA samples should be treated as if they were a patient sample and therefore must be run by personnel who would normally use the device. This provides confidence in the reliability of patient test results.
Participating in an EQA scheme allows a laboratory to gather valuable data, this data can be used in a variety of ways [1]:
EQA provides assurance to both staff and customers that testing taking place at your laboratory provides accurate and reliable results. Problems can be identified early on and corrective action can be untaken. The reliability of methods, materials, and equipment can be evaluated and training can be developed and its impact monitored.
Large laboratory groups can compare their performance with sites across their group, ensuring accuracy and consistency no matter where testing takes place.
EQA participation is often a requirement for accreditation, gaining accreditation alone has a host of benefits, not least an increased confidence in results from customers, current and potential.
Read more about accreditation: ‘The Importance of Meeting ISO 15189 Requirements’.
Point of care testing (POCT) refers to testing that is performed near or at the site of a patient with the result leading to a possible change in the care of the patient. The popularity and demand for POCT has recently seen rapid growth, this comes from the advantages including the added convenience of being able to obtain a rapid result at the patient’s bedside, thus allowing immediate action, saving time and improving the potential outcome for the patient.
Although there are many benefits of using POCT devices in terms of their convenience, these benefits are only true if the results produced are both accurate and reliable. Ensuring accuracy and reliability is the primary responsibility of Quality Control.
EQA is strongly recommended for all point of care devices and is recommended by ISO 22870, which providesspecific requirements applicable to point-of-care testing and is intended to be used in conjunction with ISO 15189.
There are many External Quality Control schemes that come in different varieties. EQA schemes can be mandatory, required either by accreditation or law. Others are voluntary and carried out by laboratories who want to ensure that they are carrying out accurate testing and improve the quality of the lab’s performance [1].
A good EQA scheme should offer:
EQA is a great tool for comparing against a peer group and maintaining an effective QC strategy, however, it has its limitations.
EQA / PT alone cannot provide a complete evaluation alone; it is important to also run third party controls regularly. You can find out about the importance of third party controls here.
EQA results can also be affected by variables not relating to patient samples, including preparation, clerical functions, matrix effects, and selection of method. The errors can appear to be a downside to EQA but it can be used as a way to evaluate staff performance as well as assay performance.
If possible, every laboratory should participate in an EQA scheme that covers all testing procedures. Laboratories need to develop a management process with the objective to assure that EQA samples are treated appropriately and in the same manner. This includes, sample handling, sample analysis, record keeping, investigating deficiencies, taking corrective actions, and communicating results with laboratory staff and management.
Problems at any stage of sample analysis can cause errors, when an error does happen, all elements of the process need to be checked. Some examples of errors:
Pre Analysis
• Incorrect sample handling during preparation, shipping or storage
• Improper storage
• The material has expired
• An error in manufacturing
Analysis
• Instrument, calibration or reagent defects
• Staff competency
• Matrix effects
• Incorrect analysis method
Post Analysis
• Report misinterpretation
• Clerical or transcription errors
• Failure to take corrective action
• Where possible, all laboratories should participate in an EQA scheme for all tests that they perform.
• EQA samples should be treated in the same way as a patient sample, using the same procedures, instruments, methods, and staff who normally perform the testing.
• EQA provides valuable resources and data to effectively maintain accurate and reliable results and should be seen as educational.
Randox offers RIQAS, the largest EQA scheme in the world with over 45,000 participants across 133 countries, offering 33 consolidated programmes. Randox also offers a range of over 90 molecular programmes for infectious disease testing with Quality Control for Molecular Diseases (QCMD).
Features and Benefits



Fully accredited to ISO/IEC 17043:2010



Consolidated programmes



High frequency reporting



The highest quality material



User-friendly reports
To learn more about RIQAS, visit the RIQAS homepage.
RIQAS
RIQAS Point of Care
QCMD
[1] WHO, Overview of External Quality Assessment (EQA). World Health Organisation, 2009.
[2] ISO 15189:2012 Medical laboratories — Requirements for quality and competence, 3rd ed. ISO, 2014.
[3] ISO 22870:2016 Point-of-care testing (POCT) — Requirements for quality and competence, 2nd ed. ISO, 2016.
RIQAS Point of Care – FAQs


RIQAS Point of Care
RIQAS
Contact Us
RTS: The Dangers of Psychoactive Substances
Psychoactive substances, formerly known as legal highs, are substances which produce the same or similar effects as many illegal drugs such as cannabis, ecstasy and cocaine. With many psychoactive substances it is extremely difficult to know their potency and therefore how they will affect the user.
They are divided into four main groups:
Sedatives – Cause reduced concentration and slowing of reactions, leading user to feel physically unsteady and lethargic.
Hallucinogenic – These substances cause psychedelic reactions which can lead the user to act erratically, putting their own safety at risk.
Stimulants – Substance temporarily causes increase in alertness and energy, while also putting a strain on the nervous system.
Synthetic Cannabinoids – Large doses can lead to life-threatening intoxication. Also effects the central nervous system causing seizures, high blood pressure, rapid heart rate and increased body temperature.
Incidents
Last month two men were arrested and jailed for four and a half years for selling psychoactive substances. The offences were committed in Scotland and eight of the men’s customers needed hospital treatment after taking the substances.
Free samples and loyalty cards were used by the men selling the psychoactive substances and two users were left in comas after taking a substance known as Psyclone. Court was told that the shops took in as much as £2,500 in sales every day.
The sister of a man who died from taking psychoactive substances said it changed her brother “mentally and physically.” BBC Scotland was told that he started dabbling with psychoactive substances because he believed they were legal. “Some of them were a substitute of heroin, others to mimic the effects of cannabis. He became very withdrawn with the family and just wanted to stay away.”
Randox Testing Services
At Randox Testing Services we reacted to the issues caused by psychoactive substances by releasing the world’s first Synthetic Cannabinoids (JWH/AM) and ‘Bath Salt’s’ (MPDV) tests in 2012. This enables companies to test for popular types of psychoactive substances and showed that it was possible to detect these substances.
However as new psychoactive substances emerged new tests also had to be created and next generation Synthetic Cannabinoids tests (UR144 and JWH250) were released in 2013. These tests represented a milestone in the fight against the highly dangerous psychoactive substances and provided a way for laboratories and companies to stay on top of the developing drug trends.
Psychoactive Substances we currently screen for;


Today, in the endless pursuit of creating innovative tests for new and emerging psychoactive substances, our expertise sets us apart from the rest of the industry. We will continue to develop our test menus and grow our range of tests.
For more information contact us today at testingservices@randox.com.
Biochip vs ELISA: Which testing platform is right for me?
Biochip Vs ELISA
Randox Toxicology’s latest video series, ‘Biochip Vs ELISA’, highlights our routine and novel ELISA products and how they differ from Biochip Array Technology.
Showcasing the journey and ongoing brand evolution of Randox Toxicology, these videos will help you to discover which method is right for you!
Episode 1: Meet ELISA
Episode 1 “Meet ELISA” uses speed reading to showcase Randox Toxicology’s extensive and ever-expanding ELISA test menu, including our range of New Psychoactive Substances, drugs of abuse, stimulants, analgesics and sedatives. Manufactured in the United Kingdom, our continuous reinvestment in research and development has enabled us to develop a range of exclusive ELISA kits such as, Mitragynine, MT-45, and U-47700 which was involved in the death of the famous singer Prince.
Our cost effective ELISA kits are the highest quality on the market and results provide excellent correlation with confirmatory methods, typically <10% CV.
Episode 2: Meet Biochip
Based on ELISA principles, Episode 2 “Meet Biochip” illustrates Biochip Array Technology as a solid-state device with discrete test sites onto which antibodies specific to different drug compounds are immobilised and stabilised. Moving away from traditional single analyte assays, Biochip Array Technology boasts cutting-edge multiplex testing capabilities for rapid and accurate drug detection from a single sample.
As the primary manufacturers of Biochip Array Technology, Randox Toxicology offer the most advanced screening technology on the market. With the world’s largest test menu capable of detecting over 500 drugs, Randox Toxicology are changing the landscape of drugs of abuse testing.
Episode 3: Biochip Vs ELISA
Episode 3 “Biochip Vs ELISA” gives you the opportunity to hear from a professional! Laura Keery our Senior Research and Development Team Leader gives you a behind the scenes look at our Biochip Array Technology and ELISA products in action at our new Science Park, answering some of those must know questions.
Episode 4: Biochip Vs ELISA 360-Degrees
If you missed it at SOFT-TIAFT 2017, our Biochip Vs ELISA 360-degree video allows you to experience Biochip and ELISA in action.
Discover which method is right for you! #biochipvselisa
For more information about our revolutionary Biochip Array Technology and ELISA kits, email info@randoxtoxicology.com or visit www.randoxtoxicology.com
Randox Testing Services | How can a policy form the basis of workplace drug & alcohol testing?
If you work in a company with safety-critical roles it is more than likely that you have some sort of workplace drug and alcohol testing policy in place. Even companies without safety-critical roles are implementing these policies to further ensure the health, safety and wellbeing of their staff.
Employers hold the responsibility to ensure employees are fully aware of the company’s rules, regulations, testing and disciplinary procedures.
The policy itself holds vital importance, providing employees with the knowledge of the standards expected of them, whilst educating themselves with information provided in a written comprehensive manner.
The importance of implementing a policy
The most important element of a workplace drug and alcohol testing policy is SAFETY. Drug and alcohol use increases the probability of workplace accidents occurring. Studies have found that employees who have alcohol problems are 2.7 times more likely to have an accident whilst at work. The main issues associated with substance misuse relate to:
- Absenteeism – it’s estimated that 17 million days of work are lost per year due to substance misuse.
- Low productivity levels – employees may reduce output in different tasks and become de-motivated.
- Inappropriate behaviour – some cases of substance abuse may lead to crime.
- Aggressive behaviour towards others – resulting in loss of employment / convictions
It’s evident that many who suffer from drug & alcohol abuse are in employment. Studies show 25% of those in employment were registered drug addicts with 3.3% of all adults aged 16-59 classified as frequent users.
Significant issues such as these provide growing concerns for employers to implement a workplace drug and alcohol policy, to ensure the welfare of each member of staff is considered. Under the Health & Safety Act 1874, employers have a responsibility to ensure the safety of their employees is fully met in order to maintain standards.
The importance of a workplace policy for drugs and alcohol can benefit employers by:
- Building relationships with employees by showing there is help and support available.
- Policies can raise awareness of issues in the business and can encourage staff members to take action if needed.
- It can reduce the number of sick employees, reduce staff turnover and increase productivity levels.
Speak with us directly
We understand that the importance of having a policy that suits the specific needs of your company. In order to fully achieve this, we offer a free policy review service, where we will review your company’s existing documentation to gain an understanding of how we can help going forward.
We are delighted to announce we will be attending the Safety & Health Expo 2018. The annual event, running from 19th – 21st June and held at the Excel London, is the UK’s largest health and safety event with over 13,500 national and international key industry professionals across construction, manufacturing, government and consulting.
By attending this prestigious event we hope to engage with a range of stakeholders to discuss how our drug & alcohol testing services can have a positive impact on your employees and business.
If you are attending this event and would like to speak with us, please stop by our stand M410 to speak with one of our experts.
Alternatively, if you would like to arrange a meeting with us prior to the event, please email us: testingservices@randox.com, and quote Safety & Health Expo 2018 at the beginning of your message.
For more information on workplace drug & alcohol testing, visit www.randoxtestingservices.com.
Drug and Alcohol Testing in the Medico-legal Market
Medico-legal testing for drugs and alcohol may be required by various professional bodies involved in child custody cases, care proceedings or child protection cases. In cases regarding divorce and children, a dispute may arise during the process of discussions involving the custody of children. In these cases drug and alcohol testing may be sought if there has been a substance abuse claim against a parent fighting for custody or visitation. In cases relating to child protection, social services may seek drug and alcohol testing if child welfare claims have been made regarding suspected substance misuse.
Normally in medico-legal cases a hair sample would be tested as it provides the longest detection window.
Why is Drug and Alcohol Testing in Medico-legal Cases Important?
Drug and alcohol testing is important to ensure child protection from the detrimental effects of parental substance misuse and to ensure they have a quality of life they deserve. In addition it is also important to enable parents the opportunity to get the help and support they need and begin rehabilitation treatment.
Doing the right thing by the child is the main priority, and where possible parent and child relationships are sought to be maintained. Drug and alcohol testing assists in these efforts and in such cases abstinence monitoring testing may be required to assess a parent’s recovery e.g. if a visitation case is being reassessed.
Our Expertise
At Randox Testing Services we provide drug and alcohol testing to all professionals within the family law and medico-legal sector. Our hair drug testing service utilises accredited testing methods and is made more cost-effective through utilising patented testing methods developed by Randox.
We understand the impact a positive result can have on a parent, child, and extended family and ensure results of the highest precision and accuracy. With over 35 years’ experience in the diagnostic industry we have gained reputation as a trusted provider.
Our drug and alcohol testing solutions are flexible and can be tailored to our customer needs with a choice of testing methods. We offer a comprehensive drugs of abuse test menu and our service also includes expert witness reporting where applicable.
Contact Us
To speak to one of our experts about hair drug testing contact enquiries@randoxtesting.com or call +44 (0) 161 741 2760. We work with companies within the medico-legal sector along with a wide range of workplaces and also private individuals.
For further information on RTS, or to arrange interviews, please contact the Randox PR team via email: randoxpr@randox.com or phone 028 9442 2413
Addressing Drugs and Alcohol in the Workplace
Within any business, companies seek to outline clear methods in which employees should act and behave whilst carrying out their roles. These rules are outlined in company workplace policies. Every business – no matter which industry it operates in – should have well-documented and comprehensive workplace policies and procedures in place.
According to the Employment Law Handbook, a workplace policy is a set of rules and principles that aims to provide guidance to managers and workers in how to behave in the workplace. They can be in place for numerous different issues – bullying, harassment, internet use, health and safety are just a few that can be implemented.
Health and Safety
As mentioned above, health and safety is an important aspect of any workplace policy. The health and well-being of the working community is of utmost importance for sustainable development. Specifically, a drug and alcohol policy is a key part of the overall health and safety policy within a company. Alcohol and drugs through their effects on health, safety, work performance and absenteeism can jeopardise productivity, deny businesses the leading edge and curtail competitiveness. Effectively implemented drug and alcohol policies will help employers in the legal duty to protect the health, safety and welfare of employees.
The need for a Drug and Alcohol Policy
Drugs and alcohol misuse can have dangerous consequences within the workplace. All organisations can benefit from an agreed policy that applies to all staff. There are wide range of statistics available to highlight the worrying impact that drugs and alcohol can have on individuals. In 2016, it was estimated that £7 billion was lost in productivity through unemployment and sickness. Furthermore, 10.8 million adults in England are drinking at levels that pose some risk to their health. A survey carried out by UK based Health and Safety Consultants Protecting.co.uk showed that; from 2,600 workers in office, factory, retail and the public sector, 85% admit to being drunk at work in the last year; not including the Christmas party. 28% of those surveyed admitted using drugs at work, including NPS (formerly legal highs) cannabis and other illegal substances.
From a legal point of view, employers have a duty of care under the Health and Safety at Work Act 1974 to ensure, as far as is reasonably practicable, the health, safety and welfare at work of employees. Also, under the Management of Health and Safety at Work Regulations 1999, to assess the risks to the health and safety of employees. If an employer knowingly allows an employee under the influence of drug misuse to continue working and his/her behaviour places the employee or others at risk, they may face prosecution.
Advantages of having Policies in place
Having well-developed policies and procedures can provide a range of benefits to an organisation. An effectively implemented drug and alcohol policy will ensure a clear understanding within the workplace of the rules relating to drugs and alcohol. It will also provide a greater awareness in workplaces of the effects of drugs and alcohol an consequently early recognition. Furthermore, it ensures that the necessary structures and procedures are in place should a problem arise. An up to date policy will also provide assurance that key staff have been trained to understand the issues involved and have the necessary skills to deal with any problems should they arise.
How can Randox Testing Services help?
At Randox Testing Services we offer a comprehensive consultancy service to help employers create, an effective substance misuse policy. By providing this service we offer practical advice, guidance and support in composing a substance misuse policy.
Our confidential policy review service provides assistance to employers with an existing substance misuse policy. With this service, we help to modify existing documents to ensure it is legally viable and can withstand challenge in court.
For more information on our comprehensive consultancy service, visit our website: www.randoxtestingservices.com or contact us by emailing testingservices@randox.com.
To read more on workplace policies and their importance within an organisation, click here.
Cocaine on the Rise
Newly emerged figures from Public Health England have documented that the UK’s current approach to drug treatment has failed to reduce drug related deaths. With UK drug abuse now at an all-time high, 2017 saw a 23% increase in treatment presentations for crack cocaine use, according to The Conversation. An additional article by the Business Insider UK reported that seizures related to cocaine in Britain are now at their highest since 2008.
Crack cocaine is a powerful stimulant designed to temporarily speed up the mind and body. Freebase cocaine (powder cocaine) and crack cocaine (rock form cocaine) can both be smoked to reach the brain quicker, whilst snorting the substance causes a slower effect. A very addictive substance, cocaine is reported to make a user over confident and careless with risks including, breathing and mental health problems, depression and the risk of an overdose related death. When taken in conjunction with alcohol the dangers of cocaine are increased, as the mixture produces the toxic chemical, cocaethylene.
The Conversation highlighted “Cuts to drug treatment budgets are extremely shortsighted. Not only do effective services save lives, they reduce the spread of blood-borne viruses, including HIV. About half of people who inject drugs have hepatitis C. Getting them into treatment is an essential part of plans to eliminate the disease.” At Randox Toxicology we offer the most comprehensive drugs of abuse test menu across multiple matrices. Our DoA ULTRA panel detects up to 20 targeted drugs, offering the largest cross-reactivity profile of over 240 analytes, including Benzoylecgonine/Cocaine. Benzoylecgonine is the most common metabolite measured in urine drug screens to detect cocaine. Using our revolutionary Biochip Array Technology, Randox Toxicology provide cutting-edge multiplex testing capabilities for rapid and accurate drug detection from a single sample.
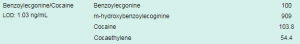
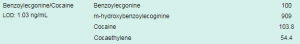
The price of cocaine has fallen by 13% since 2007 according to Business Insider UK. Price trends in addition to new and emerging UK supply routes have made the drug more appealing and readily available. Whilst the average age group using cocaine is 35 years old, a 30% increase has been seen in young people under the age of 25 years old. A rise which has not been witnessed in a decade.
The increase of cocaine use has shown how vital drug treatment is in responding to the ever-changing market, with services needing to adapt quickly to the needs of varied groups. Amidst the ongoing cocaine problem, Randox Toxicology are leading the way in developing new tests through significant research and development.
For further information on how Randox Toxicology are fighting drugs of abuse, email info@randoxtoxicology.com
Diabetes – World Diabetes Day (14th Nov 2017)
World Diabetes Day
With World Diabetes Day on Tuesday 14th November 2017, we take a look at what diabetes is and why quality control is so important.
What is Diabetes?
Diabetes is a life-long condition which occurs when the glucose level in the blood is too high because it can’t enter the body’s cells to be used as fuel. There are two types of diabetes: type 1 and type 2. They are distinct conditions and must be treated and managed differently.
Type 1 Diabetes
Type one diabetes is an autoimmune condition in which the body attacks insulin-producing cells, this causes a lack of insulin, leading to an increased blood glucose level. Around 10% of people with diabetes has type 1.
Type 2 Diabetes
A mixture of genetic and environmental factors causes type 2 diabetes. The body doesn’t make enough insulin or the insulin it does create does not work correctly, leading to a glucose build up in the blood. It’s thought that up to 58% of type 2 diabetes can be prevented or delayed through healthy lifestyle choices.
Role of Quality Control
Quality control plays a crucial role in ensuring accurate and reliable diabetes monitoring. 70% of medical decisions are based on a laboratory test result and QC is vital in ensuring the results the laboratory report are both accurate and reliable.
Want to know what makes a good HbA1c control? Read on to find out.
Clinically Relevant Levels
In the diagnosis of diabetes, glycated haemoglobin (HbA1c) in blood provides an indication of average blood glucose levels in the previous three months. HbA1c is the recommended standard of care for type 2 diabetes monitoring. HbA1c is measured using the range below:
HbA1c – Clinically Relevant Levels
| HbA1c | mmol/mol | % |
|---|---|---|
| Normal | Below 42 mmol/mol | Below 6.0% |
| Prediabetes | 42 to 47 mmol/mol | 6.0% to 6.4% |
| Diabetes | 48 mmol/mol or over | 6.5% or over |
It is important to assess the full clinical range of an assay, i.e. the range between the lowest and highest results which can be reliably reported. 48 mmol/mol is the cut-off for diabetes diagnosis, it is crucial that this can be measured accurately because any inaccuracy could mean the difference between being diagnosed and treated and not.
In terms of accreditation, ISO 15189:2012 states, ‘The laboratory should choose concentrations of control materials wherever possible, especially at or near clinical decision values, which ensure the validity of decisions made’.
Benefits of Third Party Controls
The importance of third party controls is evident. Third party controls can help identify instrument, reagent, and procedural errors. Unchecked these errors could lead to incorrect patient results, further leading to misdiagnosis.
Third party quality control material has not been designed or optimised for use with any instrument, kit, or method. This complete independence enables the quality control material to closely mirror the performance of patient samples, and in doing so, provide an unbiased, independent assessment of analytical performance across multiple platforms.
Again, in terms of accreditation, ISO 15189 states – “use of independent third party control material should be considered, either instead of, or in addition to, any control materials supplied by the reagent or instrument manufacturer.”
Many laboratories perform HbA1c testing on a dedicated machine and as a result, are not always using a third party control.
Controlling Waste
Wastage is a common issue when running HbA1c due to the pre-treatment step required for many HbA1c controls and poor stability of some controls on the market. Look out for controls with an extended open vial stability to help reduce waste and keep costs low.
How can Randox help?
To help you get your QC in check for World Diabetes Day, Randox Acusera HbA1c control contains both HbA1c and Total Haemoglobin, with a reconstituted stability of 4 weeks to reduce waste and reduce costs. To find out more about our HbA1c control visit the page using the button below or fill out the form above.


References
Diabetes: The basics. (2017). Diabetes UK. Retrieved 3 November 2017, from https://www.diabetes.org.uk/diabetes-the-basics
Khan, H et al. (2016). Significance of HbA1c Test in Diagnosis and Prognosis of Diabetic Patients. Biomarker Insights, 95. http://dx.doi.org/10.4137/bmi.s38440
NHS cancer testing service at ‘tipping point’
A stark warning has been issued this week by Cancer Research UK (CRUK) that NHS cancer testing services are at tipping point, caused by increased demand and a lack of capacity.
Tackling this is essential, according to pathology expert Professor Manual Salto-Tellez, “We need to act now before this situation gets worse. It’s vital that patients are diagnosed at an early stage when treatment is more likely to be successful.”
CRUK says the UK’s cancer survival falls behind that of other European countries and is urging an improvement in early diagnosis through diagnostic services. The importance of this is emphasised by estimates from the charity that cancer diagnoses in the UK will rise from 352,000 (2013) to 500,000 (2035).
According to the report:
- One in two people will develop cancer at some point in their lifetime
- Well-resourced testing services are crucial to early diagnosis of cancer which in turn is vital to increase survival rates
- Up to 70% of clinical decisions are based on diagnostic testing
- Pathology numbers are not growing to meet rising demand for tests
Emma Greenwood, Cancer Research UK’s director of policy, said;
“Diagnostic services, including pathology, urgently need support and investment to ensure that diagnoses aren’t delayed and patients benefit from the latest treatment. The diagnostic bottleneck will only get worse without action now and this involves addressing staff shortages in imaging, endoscopy and pathology.”
A Department of Health spokesperson said, “Early and fast diagnosis is crucial in improving patient outcomes and experience. Getting pathology test results to patients quickly is a key part of this. That’s why we have invested over £2.5bn on efficient and robust pathology services across the NHS.”
Following the publication of the report Dr Martin Crockard, Head of Molecular R&D at Randox, said;
“As populations continue to age, illnesses like cancer, stroke, diabetes and cardiovascular disease will become more common. We know this is going to have a huge impact on healthcare systems but what is yet to be determined is how they will respond.
“Currently 70% of clinical decisions are using in-vitro diagnostics and that will likely increase – therefore it’s essential that pathology services are fully supported. Better diagnostics enables clinicians to make evidence-based decisions, which delivers improved patient outcomes.”
For more information regarding our preventive health philosophy please contact our PR team via email: randoxpr@randox.com








