RX Imola: Inflammatory Biomarkers in COVID-19
RX Imola: Inflammatory Biomarkers in COVID-19
Over the course of human history, few events have had such a dramatic impact as the COVID-19 pandemic. According to the World Health Organization (WHO), as of 12th July 2023, the SARS-CoV-2 virus has claimed almost 7 million lives and figures continue to rise1. While many who become infected are only subject to mild symptoms, those who develop a more severe form of the infection are encumbered with a debilitating flu-like condition, often requiring days, if not weeks of bed rest. In a paper from June 20232, the Rx Imola was used to study C reactive protein concentrations, along with other biomarkers, in mild and severe COVID-19 patients in order to develop novel risk stratification methods for this potentially life-threatening viral infection.
The impact on healthcare services around the world cannot be understated. In developed countries, access to services for both COVID-related and other conditions took a catastrophic hit. In low-to-middle-income countries, the impact has been even more distressing, all but eliminating basic medical care in favour of combating COVID-19, partly due to inferior resources and facilities3.
In times of medical emergency, it is crucial to have an efficient and effective means of stratifying the risk to patients and a process for suitably categorising those into the least and most at risk of severe complications or death. Due to the rate at which COVID-19 spread, unfortunately, the world lacked these mechanisms for SARS-CoV-2, resulting in mass hospital overpopulation, cancelled appointments for other life-threatening conditions and ultimately the staggering mortality statistics we’ve been bombarded with since January 2022. This prompted an unprecedented surge in medical research and major advances in testing capabilities, giving us new methods of detecting SARS-CoV-2 and determining the risk posed to individuals.
One such investigation, by Paranga et al., (2023) studied a total of 13 biomarkers to determine which could accurately differentiate mild, moderate, and severe cases and identify biomarkers which were good predictors of fatality2. C reactive protein (CRP) was the best-described biomarker relating to COVID-19 throughout the pandemic. This paper compares it to 12 other biomarkers including suPAR, sTREM-1, ferritin, MCP-1 and Lactate dehydrogenase. Of these, it was discovered that CRP was clearly the most effective biomarker for differentiating mild from severe cases, with concentrations in those with severe infection being, on average, 45% higher than in those with mild symptoms2. Additionally, the authors discovered that CRP levels were not significantly affected by age, a factor known to affect the inflammation and immune responses, providing a powerful and inclusive risk stratification tool. Some of the additional conclusions drawn from this paper can be seen below2:
- Lactate dehydrogenase, sTREM-1 and HGF were good predictors of mortality in COVID-19.
- suPAR was identified as a crucial molecule in characterising Delta variant infection and mortality.
- The initial values of inflammatory biomarkers were good to excellent predictors of disease severity in COVID-19 patients.
- Disease severity and mortality are associated with a higher rate of comorbidities including thrombocytopenia and other blood diseases, circulatory and respiratory system diseases and liver diseases such as cirrhosis.
So, what is CRP and how does it become elevated in response to a SARS-CoV-2 infection? CRP is a non-specific, acute-phase protein, meaning its concentration is altered in response to inflammation4. The acute respiratory distress syndrome induced by SARS-CoV-2 is, in part, a result of the hyperinflammation caused by the virus2. CRP is a well-characterised inflammatory biomarker and is therefore well-suited for identification and risk stratification in an emerging disease.
This investigation2 utilised the RX Imola, a rapid, comprehensive clinical chemistry platform, to quantify CRP. With the RX Imola, laboratories can gain access to the world’s largest clinical chemistry test menu covering routine chemistries as well as specific proteins, lipids, and more providing a cost-effective and user-friendly platform. With 60 cooled reagent positions and a sample carousel with 20 cooled positions for controls and calibrators, the RX Imola is an ideal solution for small to medium-throughput laboratories seeking an innovative and reliable clinical chemistry system. Randox also supplies suitable, high-quality reagents, and through Acusera, state-of-the-art controls and calibrators, completing the clinical chemistry portfolio.
References
1. World Health Organisation. WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/.
2. Paranga TG, Pavel-Tanasa M, Constantinescu D, et al. Comparison of C-reactive protein with distinct hyperinflammatory biomarkers in association with COVID-19 severity, mortality and SARS-CoV-2 variants. Front Immunol. 2023;14. doi:10.3389/fimmu.2023.1213246
3. Jain P. Impact of COVID-19 Pandemic on Global Healthcare Systems and the role of a new era of global collaborations. Sushruta Journal of Health Policy & Opinion. 2021;14(3):1-5. doi:10.38192/14.3.2
4. Nehring S. C Reactive Protein . https://www.statpearls.com/articlelibrary/viewarticle/18744/.
World Hepatitis Day 2023
Introduction
World Hepatitis Day, observed on July 28th, serves as a crucial reminder of the ongoing battle against hepatitis (HBV), a viral infection that affects millions of people worldwide. In 2019, it was estimated that 296 million people were living with chronic hepatitis B, resulting in over 800,000 fatalities1. In this article, we will delve into the intricate mechanisms behind hepatitis, explore the viral species responsible for its occurrence, discuss methods for diagnosis, and shed light on treatment and management strategies.
Understanding Hepatitis
Hepatitis refers to the inflammation of the liver, often caused by viral infections. Among the primary hepatitis viruses are Hepatitis A, B, C, D, and E, each with distinct modes of transmission and characteristics2.
Mechanisms of Hepatitis Infection
Hepatitis A and E: Hepatitis A and E viruses are primarily transmitted via the faecal-oral route, often through contaminated food or water. Ingestion of these viruses leads to acute infection, and while self-limiting in most cases, they can cause significant morbidity and mortality in certain populations5,6.
Hepatitis B, C, and D: Hepatitis B, C, and D viruses are predominantly spread through blood and bodily fluids. Hepatitis B can also be transmitted from mother to child during childbirth which in endemic areas, HBV infection from mother to child transmission accounted for approximately half of chronic infections. These viruses can cause chronic infections, leading to long-term liver damage, cirrhosis, and an increased risk of hepatocellular carcinoma7,8.
Diagnosis of Hepatitis
Accurate and timely diagnosis of hepatitis is crucial for appropriate management. Diagnostic methods include:
Serology: Serological tests, such as enzyme immunoassays, are employed to detect specific viral antigens or antibodies in blood samples, aiding in the identification of different hepatitis viruses and determining the stage of infection9.
Nucleic Acid Testing: Highly sensitive molecular techniques like polymerase chain reaction (PCR) enable the detection and quantification of viral genetic material, aiding in the diagnosis and monitoring of chronic hepatitis10.
Treatment and Management of Hepatitis
The management of hepatitis depends on several factors, including the virus involved, the stage of infection, the presence of co-infections, and the individual patient’s health status. Treatment strategies encompass:
Antiviral Medications: For hepatitis B and C, antiviral drugs such as interferons and direct-acting antivirals have revolutionized the treatment landscape, offering higher cure rates and improved outcomes11,12.
Supportive Care: Hepatitis patients may require supportive care to alleviate symptoms, maintain proper nutrition, and manage complications. Vaccination against hepatitis A and B is highly recommended for prevention13.
Liver Transplantation: In cases of end-stage liver disease or hepatocellular carcinoma resulting from chronic hepatitis, liver transplantation may be considered a lifesaving option14.
Randox Hepatitis Solutions
Acusera
Acusera provides a range of positive and negative serology controls comprising various infectious diseases including Hepatitis. The table below details the suitable controls, and more information can be found on our website: Serology Quality Controls – Randox Laboratories

RIQAS
The RIQAS HIV/Hepatitis EQA programme is designed to monitor the performance of tests used to detect HIV/Hepatitis antibodies and specific antigens. All samples are conveniently supplied liquid ready-to-use and are suitable for qualitative methods of analysis.
Parameters:
- Anti-HIV-1
- Anti-HCV
- Anti-HTLV-II
- HBsAg
- Anti-HIV-2
- Anti-HBc
- Anti-HTLV-1&2 (combined)
- Anti-HIV-1&2 (combined)
- Anti-HTLV-I
- Anti-CMV
- Anti-HAV IgM
- Anti-HAV (Total)
- Anti-HBc (Total)
- Anti-HBe (Total)
- Anti-HBs (Total)
- P24
For more information, please visit our website at: HIV Hepatitis EQA | RIQAS (randox.com)
Qnostics
Monitoring for the presence of Blood Borne Virus (BBV) nucleic acid is an essential parameter in guiding clinical treatment and patient outcomes. The use of appropriate quality control measures is important in ensuring the appropriate daily performance of the molecular assay used in the laboratory independent of the technology.
Qnostics’ Blood Borne Virus Molecular Controls comprises a range of pathogens which are classically detected directly from the blood including those related to hepatitis. The table below lists the Qnostics products related to hepatitis testing. For more information visit our website: Qnostics | Molecular Infectious Disease Controls – Randox Laboratories

QCMD
QCMD is a world-leading External Quality Assessment (EQA) / Proficiency Testing (PT) scheme, dedicated to improving the quality of molecular diagnostic assays used in the detection of infectious diseases. With an extensive database of over 2000 participants in over 100 countries, QCMD is one of the largest providers of molecular EQA in the field of molecular diagnostics. QCMD programmes related to hepatitis testing are listed below:
- HBV Drug resistance Typing EQA programme.
- HCV Drug resistance Typing EQA programme.
- Hepatitis B Virus DNA EQA Programme
- Hepatitis B Virus Dried Blood Spot EQA Pilot Study
- Hepatitis B virus Genotype EQA Programme
- Hepatitis C Virus Dried Blood Spot EQA Pilot Study
- Hepatitis C Virus RNA EQA Programme
- Hepatitis C virus Genotype EQA Programme
- Hepatitis D Virus EQA Programme
- Hepatitis E virus RNA EQA Programme
For more information on any of these EQA programmes please visit: QCMD – Molecular EQA Scheme | Randox Quality Control
Conclusion
World Hepatitis Day serves as a reminder of the global impact of hepatitis and the urgent need to raise awareness, prevent transmission, and improve the diagnosis and management of this disease. By understanding the mechanisms, bacterial species involved, diagnostic techniques, and treatment approaches, we can work towards a future free from the burden of hepatitis. Let us unite in our efforts to combat this disease and strive for a healthier world.
If you’d like to find out more about hepatitis or the diagnosis and testing of hepatitis, please visit our website. If you’d like more information on how Randox can improve hepatitis testing in your laboratory, please reach out to marketing@randox.com.
References
- World Health Organization. World Health Statistics 2023. World Health Organization; 2023. https://www.who.int/publications/i/item/9789240074323
- World Health Organization. Hepatitis. https://www.who.int/news-room/fact-sheets/detail/hepatitis-a. Published 2017. Accessed June 9, 2023.
- Wan Z, Wang X. Bacterial Hepatitis. In: Encyclopedia of Medical Microbiology. Elsevier; 2020:110-117.
- Russo TA, McFadden DC. Bacterial and fungal infections in patients with cirrhosis. Clin Liver Dis. 2019;14(2):71-74.
- World Health Organization. Hepatitis E. https://www.who.int/news-room/fact-sheets/detail/hepatitis-e. Published 2018. Accessed June 9, 2023.
- Rakesh S, Pekamwar SS. Hepatitis A. In: StatPearls [Internet]. StatPearls Publishing; 2020.
- World Health Organization. Hepatitis B. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b. Published 2021. Accessed June 9, 2023.
- World Health Organization. Hepatitis D. https://www.who.int/news-room/fact-sheets/detail/hepatitis-d. Published 2021. Accessed June 9, 2023.
- Alfaresi MS, Elkoush AA, Khan AS. Serological diagnosis of viral hepatitis. J Clin Transl Hepatol. 2017;5(4):343-359.
- European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C. J Hepatol. 2017;66(1):153-194.
- European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370-398.
- Vermehren J, Sarrazin C. New HCV therapies on the horizon. Clin Microbiol Infect. 2011;17(2):122-134.
- World Health Organization. Hepatitis A. https://www.who.int/news-room/fact-sheets/detail/hepatitis-a. Published 2020. Accessed June 9, 2023.
- Kim WR, Terrault NA. Hepatocellular carcinoma and liver transplantation. Clin Liver Dis. 2018;22(2):381-394.
Exploring the Intricacies of Bile Acids: Understanding Their Role in Metabolism and Intrahepatic Cholestasis of Pregnancy
Bile acids (BAs) are fascinating molecules that play a pivotal role in our bodies metabolic processes. From aiding in the digestion of lipids to regulating essential metabolic pathways, BAs have garnered significant interest among researchers and healthcare professionals. In this article, we will delve into the structural and functional aspects of bile acids and explore their significance in a condition called intrahepatic cholestasis of pregnancy (ICP). For additional information, we encourage you to take a look at our latest educational guide: 5th Generation Bile Acids & Intrahepatic Cholestasis of Pregnancy. So, let’s unravel the secrets of bile acids and their impact on our health!
Understanding Bile Acids
Bile acids belong to a diverse family of bile salts, characterised by their planar and amphipathic nature. They possess a hydrophilic hydroxyl and a hydrophobic methyl group, conferring their unique amphipathic properties. These properties allow bile acids to emulsify and solubilize lipids, facilitating their digestion and absorption1.
Bile acids are primarily synthesized in the liver through two pathways: the classic (neutral) pathway and the alternate (acidic) pathway. The classic pathway involves the hydroxylation of cholesterol, while the alternate pathway utilizes oxysterols as precursors. These pathways produce primary bile acids, which are further modified to generate secondary and tertiary bile acids2.
Importance of Bile Acids in Metabolism
Bile acids serve multiple functions in our bodies. Firstly, they emulsify dietary fats, breaking them down into smaller droplets that can be efficiently digested by pancreatic enzymes. Additionally, bile acids are crucial for the absorption of fat-soluble vitamins, such as vitamins A, D, E, and K. These vitamins are incorporated into micelles, facilitated by the presence of bile acids, enabling their uptake3.
Furthermore, bile acids exhibit signalling activity through various receptors, influencing metabolic responses. One key receptor associated with bile acid metabolism is the Farnesoid X receptor (FXR). Activation of FXR regulates bile acid synthesis, delivery, and clearance, maintaining their levels within a safe range. FXR also modulates lipid transport and metabolism, as well as hepatic gluconeogenesis. Another important receptor is TGR5, which influences vasodilation, gallbladder function, and exerts anti-inflammatory effects1.

Intrahepatic Cholestasis of Pregnancy
During pregnancy, the metabolic processes in the liver undergo significant adaptations to accommodate the growing foetus. One condition that can arise during pregnancy is intrahepatic cholestasis, commonly known as ICP. It is a multifactorial disorder characterised by elevated levels of bile acids in the blood, particularly chenodeoxycholic acid (CDCA) and cholic acid (CA)4.
ICP manifests in the second or third trimester and can lead to various symptoms such as pruritus (itching), abnormal liver enzyme levels, jaundice, abdominal pain, and depression. The exact mechanisms underlying ICP are not fully understood, but it is believed that elevated bile acid levels may have adverse effects on the cardiovascular system of the foetus, potentially leading to stillbirth or preterm birth5.
The detection and monitoring of ICP are essential for managing the condition and ensuring the well-being of both the mother and the foetus. Total bile acid (TBA) concentration is a commonly measured parameter to assess the severity of ICP. Monitoring TBA levels can aid in identifying potential risks and enabling timely interventions5.
Introducing the 5th Generation Total Bile Acids Assay
To facilitate the accurate quantification of total bile acids in serum and plasma, the 5th Generation Total Bile Acids Assay has emerged as a reliable and advanced diagnostic tool. This assay utilizes a highly sensitive enzymatic cycling method to measure total bile acid levels, providing precise and reproducible results. With its improved sensitivity and specificity, the 5th Generation Total Bile Acids Assay offers a valuable tool for the early detection and monitoring of intrahepatic cholestasis of pregnancy.
The assay is easy to use and can be incorporated into routine laboratory workflows. It requires a small sample volume, making it convenient for both patients and healthcare professionals. The assay provides rapid results, allowing for prompt diagnosis and timely intervention when necessary.
By accurately quantifying total bile acid levels, the 5th Generation Total Bile Acids Assay aids in assessing the severity of ICP and monitoring the response to treatment. This information is vital for guiding clinical decisions and optimizing patient care during pregnancy.
Furthermore, the assay can contribute to ongoing research on bile acids and their role in ICP. By analysing a larger population and monitoring the dynamics of bile acid levels, researchers can gain deeper insights into the mechanisms underlying this condition and explore potential therapeutic targets.
Assay Principle
Two reactions are combined in this kinetic enzyme cycling method. In the first reaction, bile acids are oxidised by 3-α hydroxysteroid dehydrogenase with the subsequent reduction of Thio-NAD to Thio-NADH. In the second reaction, the oxidised bile acids are reduced by the same enzyme with the subsequent oxidation of NADH to NAD. The rate of formation of Thio-NADH is determined by measuring the specific absorbance change at 405nm. Enzyme cycling means multiple Thio-NADH molecules are generated from each bile acid molecule giving rise to a much larger absorbance change, increasing the sensitivity of the assay.

In conclusion, understanding the intricacies of bile acids is essential for comprehending their impact on our metabolism and health. Intrahepatic cholestasis of pregnancy is a condition that warrants attention, and accurate measurement of total bile acid levels is crucial for its diagnosis and management. The 5th Generation Total Bile Acids Assay offers an advanced and reliable solution for assessing bile acid levels, enabling timely interventions, and improving patient outcomes. With ongoing research and advancements in diagnostic techniques, we can continue to unravel the complexities of bile acids and enhance our understanding of their role in health and disease.
Don’t underestimate the strength of knowledge and awareness. Empower yourself, stay informed, and prioritize your health and well-being!
If you’d like to learn more about Bile Acids and ICP we encourage you to read our new educational guide, 5th Generation Bile Acids & The Importance of Of Intrahepatic Cholestasis of Pregnancy
If you would like an additional information on our 5th Generation Total Bile Acids Assay, or anything else, don’t hesitate to reach out the marketing@randox.com. Additionally, feel free to visit our Reagent resource hub where you will find all of our brochures, support tools and a collection of educational material, to aid you in maintaining the highest possible levels of quality.
References
- McGlone ER, Bloom SR. Bile acids and the metabolic syndrome. Annals of Clinical Biochemistry. 2019;56(3):326-337. doi:https://doi.org/10.1177/0004563218817798
- Chiang JYL, Ferrell JM. Bile Acid Metabolism in Liver Pathobiology. Gene Expression. 2018;18(2):71-87. doi:https://doi.org/10.3727/105221618×15156018385515
- Chiang JYL. Bile Acid Metabolism and Signaling. Comprehensive Physiology. 2013;3(3). doi:https://doi.org/10.1002/cphy.c120023
- Di Mascio D, Quist-Nelson J, Riegel M, et al. Perinatal death by bile acid levels in intrahepatic cholestasis of pregnancy: a systematic review. The Journal of Maternal-Fetal & Neonatal Medicine. Published online November 19, 2019:1-9. doi:https://doi.org/10.1080/14767058.2019.1685965
- Piechota J, Jelski W. Intrahepatic Cholestasis in Pregnancy: Review of the Literature. Journal of Clinical Medicine. 2020;9(5):1361. doi:https://doi.org/10.3390/jcm9051361
A Comprehensive Guide to External Quality Assessment Programmes
The importance of External Quality Assessment (EQA) programmes in the realm of medical laboratories is beyond dispute. These programmes serve as external control mechanisms, underpinning the accuracy and reliability of diagnostic tests carried out by laboratories across the globe. By participating in EQA programmes, laboratories gain the ability to monitor their proficiency, identify areas for improvement, enhance their analytical performance, and above all, ensure top-tier patient care.
Today, we find ourselves faced with a multitude of EQA programmes, each touting its own, unique features and benefits. Therefore, the question that naturally follows is – how do you choose the right EQA programme for your laboratory?
Understand Your Laboratory’s Requirements
The first step towards selecting an EQA programme is to clearly understand the requirements of your laboratory. These requirements could encompass the range of tests performed, the desired frequency of assessment, and the specific areas where your lab wishes to improve
Examine the EQA Programmes
The next step is to critically examine each EQA programme. Look at the range of tests they cover, the frequency of their assessments, the type of samples they use, and their approach towards feedback and improvement.
Reporting
One of the most critical aspects of an EQA programme is the results reporting mechanism. This mechanism should provide comprehensive and constructive feedback, highlighting areas of improvement, and offering guidance on how to enhance performance. It is also essential to consider the frequency of reporting. More frequent reporting allows laboratories to identify problems and implement corrective actions swiftly, aiding in the continuous improvement of a laboratory and the confident delivery of accurate patient results.
Accreditation
The accreditation of the EQA programme should also be evaluated. Superior programmes are accredited to ISO17043:2010. Participation in an accredited EQA programme is mandatory under ISO15189:2022 accreditation. Choosing a scheme accredited to ISO17043 ensures that the programme has been rigorously evaluated and meets the necessary criteria of a high-quality EQA programme.
Cost-effectiveness
The cost of the EQA programme should be compared to the benefits your laboratory will reap from participating in the scheme. Although cost should not be the sole determining factor, it’s a crucial element to consider. Factors such as consolidation and number of registrations are key areas where many providers differ.
Customer Support
Finally, it’s vital to consider the customer support provided by the EQA programme. Adequate support will ensure that any issues or queries are addressed in a timely and efficient manner
Our latest educational guide Choosing the Right EQA Programme has been constructed to help you with this decision. Providing more detail on the points discussed above and more, this guide displays how the world-renowned RIQAS EQA programmes can help you maximise the accuracy of your laboratory results and achieve ISO15189:2022 accreditation.
In conclusion, selecting the right EQA programme requires a careful and thorough evaluation of several factors. By taking the time to understand your laboratory’s needs, scrutinising each EQA programme, and considering factors such as reporting, accreditation, cost, and customer support, you can make a well-informed decision that will significantly enhance the proficiency of your laboratory and the quality of patient care.
Remember, the primary objective of an EQA programme is to help your laboratory improve. Therefore, the right EQA programme for your laboratory is the one that best assists you in achieving this objective.
World’s first Xylazine assay

Randox’s newly established ELISA for xylazine is the first immunoassay test in the world for this drug
Xylazine is an analgesic drug primarily used within the veterinary industry as a tranquilizer.
It has not been approved for human use, however, in recent years it has been linked to the USAs illicit drug supply. The United States Drug Enforcement Authority (DEA) recently published a Public Safety alert about xylazine. Its primary use is as a sedative and analgesic in veterinary medicine for horses, cattle, and other large animals. However, xylazine has been reported as an adulterant in an increasing number of illicit drugs mixtures and detected in a growing number of overdose deaths in the USA, according to the DEA.
A report released by the US Centers for Disease Control and Prevention (CDC) on June 30th 2023 found that “Among 21 jurisdictions, the monthly percentage of illicitly manufactured fentanyl-involved deaths with xylazine detected increased 276% from January 2019 (2.9%) to June 2022 (10.9%).” However, they note that because of inconsistent testing, detection is still likely underestimated.
According to the CDC researchers “Medical Examiners and Coroners might differ regarding whether they consider xylazine to increase fatal overdose risk, or they might be unfamiliar with xylazine and therefore not list it on death certificates. This variation highlights the importance of collecting postmortem toxicology data on all drugs detected in overdose deaths, rather than just those listed on the death certificate.”
The CDC advises that “Expanded postmortem testing for xylazine is needed to clarify prevalence in drug supplies” and that “Routine toxicology testing for xylazine in suspected overdose cases is critical for accurate surveillance”.
Studies continue to see the drug reported as an adulterant in an increasing number of illicit drug mixtures. Commonly encountered in combination with fentanyl but xylazine has also been detected in mixtures containing cocaine, heroin, and a variety of other drugs.
Unlike fentanyl, xylazine is not an opioid, naloxone (Narcan) does not reverse its effects. As such this dangerous combination of toxic drugs can lead to fatal accidental overdoses in users.
The severity of its side effects remain a concern: depressed breathing, blood pressure, heart rate and body temperature to critical levels.
Available now on the Evidence MultiSTAT!
Randox Toxicology continue to lead in new test development within the ever-changing drugs of abuse market. Our newly established test for xylazine is the first immunoassay test in the world for this drug of abuse.
Simultaneously screen two samples for up to 29 drugs of abuse, including xylazine, on our ToxPlex Array in just 31 minutes!
About Randox Toxicology
With over 40 years’ experience in the diagnostic market, Randox Toxicology is dedicated to advancing forensic, clinical and workplace toxicology.
Placing a heavy focus on new product R&D has led to the development of technology at the forefront of advanced global diagnostics.
A market leader in the development of new assays and technology in the field of toxicology, Randox aim to minimise laboratory workflow constraints whilst maximising the scope of quality drug detection.
With the world’s largest toxicology test menu, screening for over 600 drugs and drug metabolites, our range of versatile analysers provide toxicology solutions for both high and low throughput laboratories.
For more information, contact Randox Toxicology at info@randoxoxicology.com.
Prostate-specific Antigen & Prostate Cancer
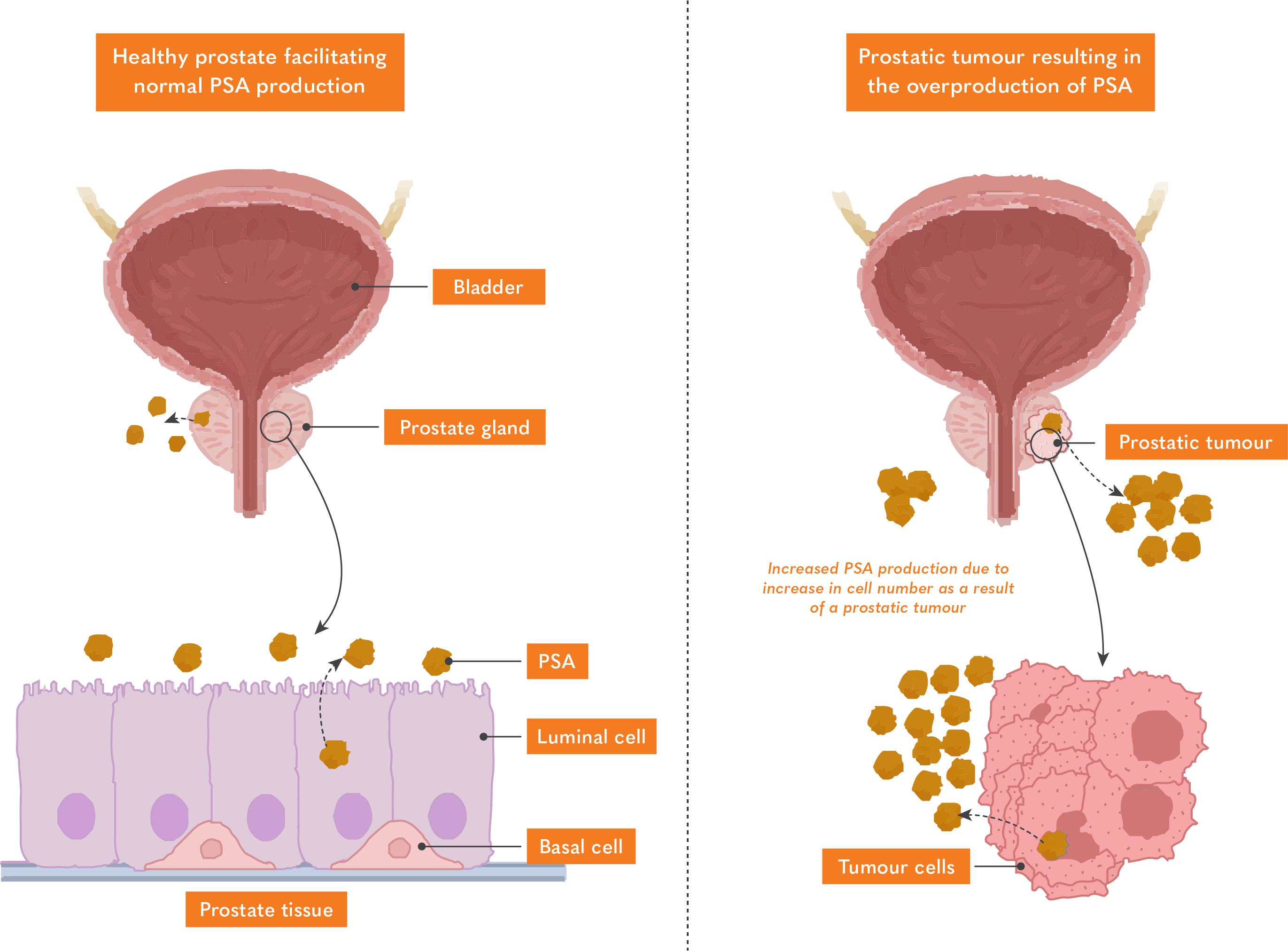
Prostate cancer is the most common form of cancer in men. In the UK, 1 in every 8 men will be diagnosed with the condition within their lifetime, resulting in around 12’000 deaths per year1. Prostate-specific antigen is a major protease found in semen which functions to cleave semeogelins into smaller polypeptides resulting in the liquefication of semen2.
This week, we had the pleasure of welcoming Dr Floris Helmich, who discussed laboratory imprecision relating to Prostate-specific antigen (PSA) and prostate cancer in our latest webinar. Dr Helmich took the time out of his busy schedule to present his experience in PSA quantification and the importance of quality control in yielding accurate and precise results as well as discussing some of the experimental techniques he has found useful in identifying the source of bias laboratory testing. Dr Helmich also discussed the ambiguity relating to reporting ranges and how bias can affect the results of laboratory PSA testing.
What is PSA?
PSA is an enzyme produced by the prostate ductal and acinar epithelium where it is secreted into the lumen before it is used to liquefy semen. Once PSA enters circulation, most are bound to protease inhibitors, however, some remain inactive and circulate in the lumen as free PSA2.
PSA levels in men vary depending on their age. Typically, men between the ages of 50 and 69 should have a PSA level below 3ng/ml. If the PSA concentration exceeds 3ng/ml, it could be a potential indicator of prostate cancer3. However, the challenge with using PSA as the sole monitoring method for prostate cancer is the relatively high false positive rate associated with it. A higher PSA concentration can also be attributed to conditions such as an enlarged prostate, prostatitis, or a urinary tract infection4.
Research indicates that 1 out of 4 men with elevated PSA levels will actually have prostate cancer. Additionally, it has been observed that approximately one in every seven men diagnosed with prostate cancer will maintain normal PSA levels3. These findings highlight the limitations of relying solely on PSA screening for prostate cancer diagnosis. As a result, some countries have started to limit their recommendations regarding PSA-based prostate cancer diagnosis.
In response to these limitations, other countries have chosen to maintain their recommendations for PSA testing but are augmenting the guidelines by incorporating additional criteria to ensure more accurate diagnoses.
Elevated PSA
Elevated levels of PSA should not always be automatically interpreted as a sign of prostate cancer. In older men, one common cause of elevated PSA is benign prostatic hyperplasia (enlarged prostate). Additionally, prostatitis, which refers to inflammation of the prostate, can contribute to an increase in PSA concentration3. It’s important to consider other potential factors that can lead to elevated PSA levels, such as urinary tract infections, recent sexual activity, natural age-related increases, or injury to the groin area5.
Therefore, when assessing PSA levels, it is crucial to recognize that various non-cancerous conditions can also result in elevated PSA. It is recommended to consult healthcare professionals who can evaluate the individual’s medical history, perform further diagnostic tests, and consider other clinical factors to accurately determine the underlying cause of elevated PSA and make informed decisions about the next steps in diagnosis and treatment.
Ultra-low PSA concentrations
The diagnostic accuracy of PSA concentration for prostate cancer is known to be limited. However, there is a clear association between PSA levels and prostate cancer, which confirms it as a valuable tool for risk stratification and diagnosis when used in conjunction with other established factors.
PSA testing also plays a crucial role in monitoring patients who have undergone treatment for prostate cancer. In cases where the patient is deemed cancer-free, their PSA levels should decrease to within the normal range. Following radical prostatectomy (removal of the entire prostate), PSA levels should ideally be undetectable. Post-radiotherapy, it is expected that PSA levels will reach their lowest point (nadir) within 12-18 months. However, it’s important to note that in some cases, a temporary spike in PSA concentration has been observed after radiotherapy. This spike should not be immediately interpreted as recurrent cancer, but these patients should be closely monitored.
If PSA concentrations rise above 2.0ng/ml after radiotherapy, further testing is recommended to assess the possibility of recurrent cancer. Close monitoring and additional evaluations will help healthcare professionals make accurate and timely decisions regarding the patient’s ongoing treatment and care6
Guidelines
Different countries offer varying guidance in relation to Ultra-low PSA testing. The table below details some of these recommended guidelines:
| Guidelines | Description |
| American Urology Association 7 | PSA concentrations of >0.2ng/ml, followed by a subsequent confirmatory >0.2ng/ml result should be considered biochemical recurrence. However, a cut-off of 0.4ng/ml may better predict metastatic relapse. |
| European Association of Urology8 | A detectable PSA indicating relapse should be differentiated from a clinically meaningful relapse. PSA thresholds that predict further metastasises are:
Post-RP = >0.4ng/ml Post-RT = nadir + 2ng/ml |
| Prostate Cancer Foundation1 | Post-RP = PSA 0.2ng/ml is indicative of biochemical recurrence
Post-RT = PSA nadir + 2ng/ml is indicative of biochemical recurrence |
Randox Ultra-low PSA Control
We are excited to introduce Randox’s latest innovation, the Ultra-low PSA Control, designed to assist in the precise quantification and monitoring of ultra-low levels of PSA in post-therapy prostate cancer patients. This control has been specifically optimized for use on Roche systems, ensuring exceptional performance and compatibility. Moreover, it is versatile enough to be utilized on various other platforms, making it the sole control available on the market for measuring ultra-low levels of PSA across a range of instruments.
With the Acusera Ultra-low PSA Control, healthcare professionals can achieve accurate and reliable results, enabling them to monitor the progress and treatment response of prostate cancer patients with heightened sensitivity. With a clinically relevant concentration of approximately 0.055ng/ml, this advancement in control technology contributes to enhanced patient care and supports medical professionals in making informed decisions regarding treatment adjustments or further interventions.
Randox’s commitment to innovation and precision in diagnostic solutions continues with the Ultra-low PSA Control, empowering laboratories to deliver high-quality and dependable PSA measurements, even at the ultra-low levels required for post-therapy monitoring.
Take a look at our webinar, Laboratory Imprecision in Relation to PSA and Prostate Cancer Follow-up, with Dr Floris Helmich to learn about how his clinical laboratory deals with bias at quality control relating to Ultra-low PSA quantification
If you’d like to learn more about PSA testing and prostate cancer, we encourage you to read our new educational guide, Ultra-low PSA and Prostate Cancer
If you would like an additional information on our Ultra-low PSA Control, or anything else relating to Quality Control, don’t hesitate to reach out the marketing@randox.com. Additionally, feel free to visit our QC resource hub where you will find all of our brochures, support tools and a collection of educational material, to aid you in maintaining the highest possible levels of quality.
References
- Prostate Cancer Foundation. About prostate cancer. Prostate Cancer UK. Published 2023. https://prostatecanceruk.org/prostate-information-and-support/risk-and-symptoms/about-prostate-cancer
- Balk SP, Ko YJ, Bubley GJ. Biology of Prostate-Specific Antigen. Journal of Clinical Oncology. 2003;21(2):383-391. doi:https://doi.org/10.1200/jco.2003.02.083
- NHS Choices. Should I have a PSA test? – Prostate cancer. NHS. Published 2019. https://www.nhs.uk/conditions/prostate-cancer/should-i-have-psa-test/
- Isono T, Tanaka T, Kageyama S, Yoshiki T. Structural Diversity of Cancer-related and Non-Cancer-related Prostate-specific Antigen. Clinical Chemistry. 2002;48(12):2187-2194. doi:https://doi.org/10.1093/clinchem/48.12.2187
- Mejak SL, Bayliss J, Hanks SD. Long Distance Bicycle Riding Causes Prostate-Specific Antigen to Increase in Men Aged 50 Years and Over. Steyerberg EW, ed. PLoS ONE. 2013;8(2):e56030. doi:https://doi.org/10.1371/journal.pone.0056030
- Santis D, Gillessen S, Grummet J, et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer.; 2023.
- AUA. Advanced Prostate Cancer: AUA/ASTRO/SUO Guideline (2020) – American Urological Association. www.auanet.org. Published 2023. https://www.auanet.org/guidelines-and-quality/guidelines/advanced-prostate-cancer
- Sindhwani P, Wilson CM. Prostatitis and serum prostate-specific antigen. Current Urology Reports. 2005;6(4):307-312. doi:https://doi.org/10.1007/s11934-005-0029-y

Acusera 24·7 Software Updates v3.3
Randox Quality Control is thrilled to announce the release of our latest software update for Acusera 24·7, which includes a collection of new features to enhance your user experience and create a more effective quality management system for your laboratory. This update shall take place on Tuesday 20th June 2023. Below, you’ll find details of the latest software updates and how these changes can help you improve your daily QC activities.
Events
- Users can now add an event at the assay, instrument or QC levels to allow more accurate monitoring of control events. In addition, this feature adds the capability to record reagent lot changes.
- Users now have the ability to temporarily hide all events from the interactive charts, allowing for a clearer view of QC performance over a selected timeframe.
Uncertainty of Measurement
- User can now add the uncertainty of the calibrator value to the uncertainty of measurement report to provide a more accurate assessment of uncertainty.
- Users now have the ability to hide the intraprecision data from the uncertainty of measurement report if no data has been entered for this field.
Charts
- A selection of new interactive charts have been added to this software. These charts focus on the individual results per analyte that each instrument generates over a specified time period.
Individual results
This graph displays a spread of the individual results for a single machine, per analyte generated over a specified time.

Weekly Count
This Bar Chart shows the weekly count of quality control results for a specific instrument, assay and lot.

Instrument Comparison
Users can now view a line graph, which plots the weekly mean of results from multiple instruments using the same assay and QC lot, allowing a comprehensive overview of your QC data.

If you’d like to learn more about these updates, we encourage you to watch our new Acusera 24·7 video guides: Acusera 24.7 Video Guides
This software update will be live from Tuesday 20th June 2023. To make the upgrade process as smooth as possible, we encourage Acusera 24·7 users to clear their browser cache, visit the Acusera 24·7 site, and you will be ready to avail of these new features!
If you would like an additional information on these updates, or anything else relating to Acusera 24·7, don’t hesitate to reach out the marketing@randox.com. Additionally, feel free to visit our QC resource hub where you will find all of our brochures, support tools and a collection of educational material, to aid you in maintaining the highest possible levels of quality.

Enhancing Laboratory Quality Control with Multi-Rule QC: A Comprehensive Guide
Introduction
We are thrilled to announce the release of our latest educational guide, “Understanding Multi-rule QC,” which delves into the world of laboratory quality control. Designed for laboratory professionals, this comprehensive guide aims to empower you with knowledge and strategies to ensure accurate results and uphold patient safety.
Understanding the Significance of Multi-Rule QC
Laboratory quality control is paramount in maintaining the integrity of test results. The guide begins by exploring the various causes of deviations in laboratory testing processes. From instrument malfunctions to environmental factors, we shed light on potential sources of error that can impact result accuracy.
Next, we dive into the core of the guide: Multi-rule QC. This powerful framework encompasses a series of rules that serve as a robust screening tool for identifying outliers, shifts, and trends in data. Through an in-depth exploration of rules such as 1:2s, 1:3s, 2:2s, R4s, 3:1s, 4:1s, 10x, and 7T, we unveil their underlying principles and their significance in maintaining quality control within laboratory settings.
Applying the Multi-Rule QC Approach
The guide equips laboratory professionals with practical insights on applying the Multi-rule QC approach. By examining consecutive data points, analysing trends, and detecting systematic shifts, you gain the ability to proactively address issues before they compromise result accuracy. We highlight the importance of avoiding overreliance on individual rules for result rejection, emphasizing the need to consider additional factors such as clinical relevance and method performance.
Troubleshooting Out-of-Control Events
No laboratory is immune to out-of-control events. That’s why our guide goes beyond rule implementation and delves into effective troubleshooting strategies. We provide guidance on identifying root causes, implementing corrective actions, and re-establishing control in your laboratory environment. By embracing a culture of continuous improvement, you can minimize the impact of deviations and optimize laboratory performance.
Acusera 24.7
Acusera 24.7 is a cloud-based inter-laboratory data management and peer-group reporting software designed to assist in the management of daily QC activities and aid continuous improvement in the laboratory. It includes multi-rule capabilities that can be utilized to monitor your QC data and index it as accepted, rejected, or trigger an alert, depending on the pre-defined multi-rules against which you want to check your data. These features enable the identification of nonconformities and reduce the need for laborious manual statistical analysis while enhancing the accuracy and precision of the laboratory.
Conclusion
In an era where accuracy and patient safety are paramount, the “Multi-rule QC” guide serves as an invaluable resource for laboratory professionals. By mastering the principles and applications of Multi-rule QC, you can enhance the quality control processes within your laboratory, mitigating risks and delivering reliable test results.
To explore the full potential of Multi-rule QC and embark on a journey of laboratory excellence, we invite you to download the guide today. Stay ahead of the curve and ensure the highest standards of quality and patient care in your laboratory!
You can download the Understanding Multi-rule QC Educational Guide below:
If you’d like to find out more about what we can do to help your laboratory or view our range of Internal Quality Controls, don’t hesitate to contact us at marketing@randox.com or feel free to browse the range on our website https://www.randox.com/laboratory-quality-control-acusera/.

Randox Health – helping people reach the peak of their fitness.
Sam Cairns has completed what seemed to be the impossible – overcoming physical and mental hurdles to stand on top of the world. Cairns, from Aviemore, which is situated within the Cairngorms National Park in the Highlands of Scotland, successfully summited Mount Everest with help from County Antrim based, Leading Diagnostics company, Randox Health.
Ex Team-GB biathlete, Cairns is also the founder of Fitnessat58° and the cofounder of the Lunchbox boys. To Cairns climbing Mount Everest represented the pinnacle of all his aspirations, a true test of his physical capabilities.
He successfully reached the summit on Thursday, May 18th, at 4:45 am.
He also faced elemental dangers on his journey – facing multiple avalanches along the treacherous route. Undeterred by the different physical and mental obstacle he faced along the way Cairns pressed on to complete his self-assigned challenge, defying all the odds stacked against him to accomplish the end goal of reaching the top.
In preparation for this challenge Cairns’ training regimen encompassed 3-hour conditioning sessions, 50km ultra runs, and multiple ascents of Ben Nevis within a remarkable time frame.
Cairns worked closely with Randox Health throughout his training, utilizing the use of Randox’s health kits and Everyathlete health program to optimize his training and nutrition through data-driven insights.
“I’ve worked closely with Randox Health, whose team played a huge role in this process.
“Using their health kits, I gained key insights to ensure my health was where it needed to be, allowing me to optimize my training and nutrition for maximum performance with the Everyathlete health program.
“It has over 80 data points linked to nutrition, muscle, joint, hormonal health, and body composition; I used repeat testing to monitor changes in my health data. Randox Health also generously made a financial donation towards the expedition.”
The Everyathlete health program, which aided Cairns in his training helps optimize training, as noted, and nutrition for maximum performance. Over 80 data points linked to nutrition, muscle, joint, hormonal health, and body composition measured are included to help athletes keep on track, stay motivated and monitor your health data.
For Male Athletes, there is an option to include PSA is available during booking. The Everyathlete programme is perfect for anyone who wants to be proactive about their health, establish their health baseline prior to training and track their health data throughout training.
It not only helps people reach the peak of the world but also the peak of their fitness.


Biomedical Science Day 2023
This 8th June, Randox is celebrating Biomedical Science Day and our vital role in pro-active healthcare through our Randox Health Clinics.
Biomedical Science Day celebrates the vital role biomedical scientists play in patient care through the diagnosis of infections and diseases. It is a national event organized by the Institute of Biomedical Science (IBMS), the professional body for biomedical scientists and laboratory support staff. The awareness day aims to inform the public and empower patients by telling them about practices in biomedical science and celebrating a profession that is #AtTheHeartOfHealthcare.
Biomedical science is practiced in healthcare laboratories to identify, research, monitor and treat diseases. As one of the broadest areas of modern science, it focuses on the complexity of the human body and underpins much of modern medicine.
Here at Randox we continuously celebrate all our laboratory staff on a global scale – our team consists of over 650 research scientists and engineers who work in four jurisdictions across three continents!
For over forty years, Randox laboratory staff have been contributing to our work in preventative healthcare and research into diagnostics.
Randox has many departments which all work collectively to produce results. Our laboratory and R&D scientists are continuously researching new biomarker discovery. With a driven commitment to improving health worldwide, Randox reinvest up to 25% of turnover into developing disruptive innovations in diagnostics and healthcare – providing earlier diagnosis, prognosis, and improved patient risk to reduce current invasive diagnostic methods.
















