Vivalytic | SARS-CoV-2 Rapid Dual Target

Vivalytic | SARS-CoV-2 Dual Target Rapid Test
–
Detecting E & N gene sequence for SARS-CoV-2
SARS-CoV-2 Dual Target Detection
Clinical Significance
SARS-CoV-2 dual target is a rapid real time PCR test cartridge, providing clear and concise results in a timely manner, direct at the point of care. This enables the patient to take the recommended safety precautions without delay.
The SARS-CoV-2 dual target rapid test allows for detection of both the E-gene and N-gene sequence for SARS-CoV-2 with minimal workflow required, suitable for laboratory and non-laboratory settings. The SARS-CoV-2 dual target test also contains a Human and Internal PCR control for convenience.
In collaboration with Bosch, we are proud to release not only a rapid testing solution for the detection of SARS-CoV-2 but an accelerated mass testing solution to effectively and efficiently monitor and detect viral infection from the offset with an aim of minimising the rise in infections globally. The new SARS-CoV-2 pooling test will allow users to test up to 160 samples a day and has sensitivity of 98% and a specificity of 100% – a world’s first!*
* Detailed information on the determination of analytical performance can be found in instructions for use provided with SARS-CoV-2 kit.
* Test Termination: If one of the target genes is clearly detected, the test can be terminated prematurely with the valid positive result.
Features
Sample Type: Nasopharyngeal or Oropharyngeal Swab (eNAT)
Sample Volume: 300 μl
Detection Method: Real-Time PCR
Time to result: 53 minutes
| Detectable Virus |
|---|
| SARS-CoV-2 (E-gene and N-gene sequence) |
“AWARD-WINNING DESIGN DELIVERS
AN UNCOMPLICATED USER EXPERIENCE”
Vivalytic Workflow
Intuitive engineering of Vivalytic ensures the analyser is user friendly. The process of patient sample to result comprises a very simple 4 step workflow.
To begin the test, the user scans or enters sample information. The cartridge code is then scanned into the embedded Vivalytic software. The user then adds sample into the dedicated cartridge slot, closes the lid and inserts the cartridge into the Vivalytic.
The touchscreen display will countdown the time remaining to test completion. Results will be displayed on the screen. Multiple Vivalytics can be wirelessly connected allowing the user to control multiple tests at one time all reporting to a master Vivalytic platform.
Want to know more?
Contact us or visit our COVID-19 Monitoring & Management page
Related Products
Viral Respiratory Infection Array
SARS-CoV-2 Pooling Test
Vivalytic
Vivalytic Test Menu
Rapid PCR MRSA/SA testing now available on Vivalytic
Rapid PCR MRSA/SA testing now available on Vivalytic
Providing a quick diagnosis of methicillin resistant at the point of the care, the latest addition to the Vivalytic portfolio of tests, not only provides rapid RT-PCR results in 53 minutes but differentiates whether the bacterial strain is methicillin-resistant (MRSA) or methicillin-sensitive (MSAA) which promotes targeted therapy.
MRSA is a major multi-resistant nosocomial pathogen worldwide with the WHO estimating that the mortality rate of patient infection rates is around 50% higher compared with patients who have been infected by non-resistant Staphylococcus aureus strains.1 Moreover, the extensive period of hospitalisation, morbidity, and the associated medical costs increase significantly with an MRSA infection.2
Introducing MRSA to the vivalytic portfolio can provide high quality answers, anywhere and anytime improving patient pathways and the need for care. Significantly, introducing rapid MRSA screening at both ward level, emergency settings and before hospital elective surgery procedures allow for an effective response to identifying whether the bacteria strain is methicillin-sensitive (MSSA) or -resistant.
Making a point to care, the rapid essence and speed of Vivalytic not only showcase technology but the ability to contribute to current health risks by preventing contamination, breaking the chain of infection, and again fighting the silent pandemic of antimicrobial resistance (AMR) & superbugs.
The treatment on the front line today looks at increasing empirical antibiotic prescribing and increasing drug-resistant outbreaks. AMR is growing rapidly, with superbugs threatening the ability to treat common infectious diseases appropriately. The COVID-19 pandemic has elevated concerns over AMR and antibiotic-associated adverse events, with surges in antibiotic prescribing, hospitalisations, and drug-resistant bacterial transmissions.
Speed is key here – since the result of diagnostics with culture sampling, which is the current traditional method for MRSA testing is only available after one to three days, this PCR test for the point of care is ideal as an additional tool when speed is of the essence.
Few points to note about the current Vivalytic panel for MRSA/SA detection:
- By using one single cartridge, the Vivalytic MRSA/SA test detects and differentiates between MRSA and MSSA DNA to aid in the diagnosis of MRSA infection in a speedy manner so that appropriate antibiotic treatment can be applied, and complications prevented.
- Detection Method: Real-Time PCR
- Result Time: 53 minutes
- Sample Volume: 600 μl
- Sample Type: Nasal- or oropharyngeal swab sample


| DETECTABLE DNA PATHOGENS: | SPECIFIC GENE TARGETS: |
|---|---|
| Methicillin-resistant Staphylococcus aureus (MRSA) | SCCmec/orfX junction |
| Methicillin-sensitive Staphylococcus aureus (MSSA) | mecA/ mecC, SA422 |
Making this happen, The MRSA/SA rapid test on Vivalytic by Bosch, a point of care platform brought to the market by Randox Laboratories. The Vivalytic system is a fully automated, cartridge-based platform capable of both Hi-Plex and Lo-Plex infectious disease testing. Each easy-to-use cartridge contains all necessary reagents, is fully-sealed to minimise risk and can be conveniently stored at room temperature.
The Vivalytic consolidates the full molecular workflow into a small benchtop platform, capable of extraction, PCR amplification and detection. It follows an easy 4 step process from sample entry to results and with the gold standard PCR testing. With most up to date technology, the Vivalytic has wireless connectivity, with no peripherals required, making a unique space saving and hygienic solution. Handling and utilisation are simple and medical professionals require only minimal training.


For more information on the Vivalytic, why not visit our webpage- https://www.randox.com/vivalytic-molecular-point-of-care/
For more information on our new MRSA test, please contact market@randox.com
ABOUT RANDOX
NEWS
VACANCIES
OUR PEOPLE
National STIQ Day 2022
National STIQ Day 2022
14th January, National STIQ day was launched to get people thinking about the importance of sexual health and encourage everyone to get regular health checks.
More than 30 different bacteria, viruses and parasites are known to be transmitted through sexual contact. Eight of these pathogens are linked to the greatest incidence of sexually transmitted diseases (STI’s). STI’s are spread predominantly by sexual contact, including vaginal, anal and oral sex. Some STI’s can also be transmitted from mother-to-child during pregnancy, childbirth, and breastfeeding.
Any individual can catch an STI regardless of what age they are, their sexuality or how many sexual partners they have, as it only takes one sexual encounter to increase the risk of catching an infection. The importance of regular STI testing allows individuals to get peace of mind and take control of their sexual health as many infections don’t present symptoms and it’s advised that testing is required if individuals are concerned about their health, present symptoms or change sexual partner. Regular screening can aid in detecting an infection in the early stages and help to reduce the risk of such complications.
The increase in STI’s underline the need for urgent action which many reports have highlighted the need of ongoing inaction and development of strategies to improve sexual health. It has been widely noted by the CDC that over 20 million new STI’s are detected each year. (1) Public Health England also reported that 468,342 diagnoses of STIs had been reported in England in 2019 – a 5% increase from 2018 with a 26% increase in gonorrhoea infections, 10% increase in syphilis and 7% increase in the number of consultations at national sexual health services. It’s also widely noted that chlamydia testing, which is most common in young adults has declined by 13% since 2015 and 2% of all individuals tested had received a positive diagnosis. (2) (3)
Here at Randox, we offer solutions for clinical laboratories, point of care testing solutions and home STI testing kits for convenience and discretion. Randox provides the broadest STI testing menu on the market. Detecting 10 bacterial, viral, and protozoan infections, the STI test provides a comprehensive sexual health profile. The CE-marked STI Array provides the identification of co-infections, often in asymptomatic individuals and enable antibiotic stewardship.
The Randox test presents excellent precision, specificity, sensitivity and accuracy for STI diagnoses, which reduces the risk of false reporting and unnecessary confirmatory tests. The Randox simultaneous multiplex test means smaller sample volumes are required, enabling faster throughput and rapid patient diagnosis saving time and money for clinical and laboratories.
The Randox STI Multiplex test detects the following infections:
- Chlamydia trachomatis (CT)
- Neisseria gonorrhoeae (NG)
- Trichomonas vaginalis (TV)
- Mycoplasma genitalium (MG)
- Treponema pallidum (Syphilis) (TP)
- Herpes simplex virus 1 (HSV-1)
- Herpes simplex virus 2 (HSV-2)
- Haemophilus ducreyi (HD)
- Mycoplasma hominis (MH)
- Ureaplasma urealyticum (UU)
Does your laboratory or clinic carry out STI testing? Our molecular analyser, the Bosch Vivalytic and Evidence Investigator, powered by patented Biochip Array Technology, could be the diagnostic solution you need!
Solutions for the Laboratory
54 SAMPLES ● 1 TEST ● 10 INFECTIONS
The Evidence Investigator is a compact semi-automated benchtop analyser ideal for medium throughput laboratories.
- Sample Type: Swab or Urine
- Sample Volume: 300 μl
- Detection Method: Randox Biochip Technology (end-point PCR)
- Time to Result: 5 hours 30 minutes
STI Testing at the Point of Care
1 SAMPLE ● 1 TEST ● 10 INFECTIONS
The Bosch Vivalytic enables sample to answer, cartridge based molecular diagnostics at
the point of care. Powered by Randox Biochip Technology.
- Sample Type: Swab or Urine
- Sample Volume: 300 μl
- Detection Method: Randox Biochip Technology (end-point PCR)
- Time to Result: 2 hours 30 minutes
For more information about our STI Arrays or Vivalytic email: marketing@randox.com
- https://www.cdc.gov/std/life-stages-populations/adolescents-youngadults.htm
- https://www.tht.org.uk/news/rise-stis-underlines-need-urgent-action
- Sexually transmitted infections (STIs) (who.int)
Related Products and Services
Evidence Investigator
Biosciences
ANTIBODY TEST
Vivalytic
Vivalytic | Extensive SARS-CoV-2 Pooling






Vivalytic | Extensive SARS-CoV-2 Pooling Test
–
15 Samples, 1 Cartridge
Simultaneous SARS-CoV-2 detection from up to 15 pooled samples
Extensive SARS-CoV-2 Pooling is a rapid real-time PCR test cartridge, providing clear and concise results in a timely manner, direct at the point of care on the Vivalytic. The SARS-CoV-2 pooling test with lollipop swab allows for the screening of up to 15 patient samples. One of the first rapid pooling PCR tests on the market, the Vivalytic produces results in less than 45 minutes delivering high accuracy for extensive and faster testing ideal for rapid and confirmatory SARS-CoV-2 screening in any environment.
Ideal for mass testing, the pooling could be done at the level of a ward, medical speciality, social bubble, or group of colleagues. It has potential for use in other settings, such as pre-operative screening, schools and universities, prisons, nursing homes, primary care, and large workplaces. Sample pooling allows more people to be tested quickly using fewer testing resources.


“AWARD-WINNING DESIGN DELIVERS
AN UNCOMPLICATED USER EXPERIENCE”
Vivalytic Workflow
Intuitive engineering of Vivalytic ensures the analyser is user friendly. The process of patient sample to result comprises a very simple 4 step workflow.
To begin the test, the user scans or enters sample information. The cartridge code is then scanned into the embedded Vivalytic software. The user then adds sample into the dedicated cartridge slot, closes the lid and inserts the cartridge into the Vivalytic.
The touchscreen display will countdown the time remaining to test completion. Results will be displayed on the screen. Multiple Vivalytics can be wirelessly connected allowing the user to control multiple tests at one time all reporting to a master Vivalytic platform.
Want to know more?
Contact us or visit our COVID-19 Monitoring & Management page
Related Products
Viral Respiratory Infection Array
SARS-CoV-2 Rapid Test
Vivalytic
Vivalytic Test Menu
SARS-CoV-2 Vascular & Multi-System Dysfunction Whitepaper


30 June 2021
SARS-CoV-2 Vascular & Multi-System Dysfunction Whitepaper Download
COVID-19, the disease caused by SARS-CoV-2, is an infectious disease caused by a newly discovered coronavirus. While many of whom become infected by the disease will experience mild to moderate cold or flu-like symptoms, those with health complications – such as autoimmune diseases, asthma, heart disease and diabetes – are at risk of developing serious illness and adverse outcomes.
As of September 2021, over 228 million COVID-19 cases have been confirmed worldwide, with an estimated one in six patients experiencing complications which could be life threatening, with over £116 billion spent by the UK government alone on measures to combat the disease. This drastic spending has been mirrored across the globe, with the significant economic burden expected to be suffered for generations to come.
The whitepaper provides a brief overview of the COVID-19 pandemic, before discussing vascular abnormalities and associated complications brought on by the virus, such as multi-system disfunction, acute respiratory disease syndrome (ARDS) and hepatic, renal & cardiovascular function.
Want to know more about Randox?
Contact us or visit our homepage to view more.
Our COVID-19 Products and Services
HOME TEST
ANTIBODY TEST
TRAVEL CERTIFICATE
LABORATORY TOOLS
Vivalytic | Customer Support






Vivalytic | Customer Support
–
Molecular Diagnostics at the Point of Care
Video Tutorials
For your support, we have compiled a series of informative video tutorials on how to install and operate the Vivalytic machine. You can use these guides to refresh your knowledge from prior training or to learn about specific procedures step by step.
Installation and Operational Guide
Instructional Promo Video
COVID-19 Testing Process
Want to know more?
Contact us or visit our COVID-19 Monitoring & Management page
Related Products
Vivalytic Overview
SARS-CoV-2 Rapid Test
SARS-CoV-2 Pooling Test
Vivalytic Test Menu
Vivalytic | SARS-CoV-2 Pooling Test






Vivalytic | SARS-CoV-2 Pooling Test
–
Detecting SARS-CoV-2 (COVID-19)
Detecting SARS-CoV-2 (COVID-19) from up to 5 Pooled Samples Simultaneously
Clinical Significance
SARS-CoV-2 Pooling (COVID-19) is a rapid real time PCR test cartridge, providing clear and concise results in a timely manner, direct at the point of care. This enables the patient to take the recommended safety precautions without delay. The pooling could be done at the level of a ward, medical speciality, social bubble, or group of colleagues. It has potential for use in other settings, such as pre-operative screening, schools and universities, prisons, nursing homes, primary care, and large workplaces. Sample pooling allows more people to be tested quickly using fewer testing resources.
In collaboration with Bosch, we are proud to release not only a rapid testing solution for the detection of SARS-CoV-2 (COVID-19) but an accelerated mass testing solution to effectively and efficiently monitor and detect viral infection from the offset with an aim of minimising the rise in infections globally. The new SARS-CoV-2 pooling test will allow users to test up to 160 samples a day and has sensitivity of 98% and a specificity of 100% – a world’s first!* Highly sensitive assays allow for laboratories to accurately detect low positive samples, enabling for effective identification of positive COVID-19 cases in a timely manner.
* Detailed information on the determination of analytical performance can be found in instructions for use provided with SARS-CoV-2 pooling kit.
Features
Sample Type: Nasopharyngeal or Oropharyngeal Swab
Sample Volume: 150 μL per-patient sample (If
less than 5 patient samples, supplement the remaining
volume with eNAT solution)
Detection Method: Real-Time PCR
Time to result: 44 minutes
| Detectable Pathogens |
|---|
| SARS-CoV-2 (E gene sequence) |
“AWARD-WINNING DESIGN DELIVERS
AN UNCOMPLICATED USER EXPERIENCE”
Vivalytic Workflow
Intuitive engineering of Vivalytic ensures the analyser is user friendly. The process of patient sample to result comprises a very simple 4 step workflow.
To begin the test, the user scans or enters sample information. The cartridge code is then scanned into the embedded Vivalytic software. The user then adds sample into the dedicated cartridge slot, closes the lid and inserts the cartridge into the Vivalytic.
The touchscreen display will countdown the time remaining to test completion. Results will be displayed on the screen. Multiple Vivalytics can be wirelessly connected allowing the user to control multiple tests at one time all reporting to a master Vivalytic platform.
Want to know more?
Contact us or visit our COVID-19 Monitoring & Management page
Related Products
Viral Respiratory Infection Array
SARS-CoV-2 Rapid Test
Vivalytic
Vivalytic Test Menu
Vivalytic | Sexually Transmitted Infection Array






Vivalytic | Sexually Transmitted Infection Array
–
1 Sample | 1 Test | 10 Infections
Comprehensive Sexual Health Profile
Clinical Significance
The Sexually Transmitted Infection (STI) array is the broadest multiplex cartridge-based STI test on the market simultaneously detecting 7 bacterial, 2 viral and 1 protozoan infection for a comprehensive sexual health profile.
Globally, each day over 1 million people are exposed to an STI, many of who will not know they have an infection as many STI’s are asymptomatic whilst some individuals will display similar or overlapping symptoms, therefore co-infections may remain undiagnosed. Designed to offer a complete sexual health profile with an aim of prevention and control, the Vivalytic STI array can be used to diagnose existing infections whilst any identifying co-infections.
To find out more about the global economic burden of STIs and the impact of the COVID-19 pandemic on sexual health, download our most recent whitepaper that presents issues such as AMR, the development of super-gonorrhoea, and the challenges faced in a social and public concept due to the COVID-19 pandemic.
Features
Sample Type: Swab or Urine (eNAT, Roche COBAS medium, or PBS)
Sample Volume: 300 μl
Detection Method: Randox Biochip Technology (end-point PCR)
Time to result: 2 hours 20 minutes
| Detectable Infections | |
|---|---|
| Chlamydia trachomatis (CT) | Herpes simplex virus 1 (HSV-1) |
| Neisseria gonorrhoeae (NG) | Herpes simplex virus 2 (HSV-2) |
| Trichomonas vaginalis (TV) | Haemophilus ducreyi (HD) |
| Mycoplasma genitalium (MG) | Mycoplasma hominis (MH) |
| Treponema pallidum (Syphilis) (TP) | Ureaplasma urealyticum (UU) |
“AWARD-WINNING DESIGN DELIVERS
AN UNCOMPLICATED USER EXPERIENCE”
Vivalytic Workflow
Intuitive engineering of Vivalytic ensures the analyser is user friendly. The process of patient sample to result comprises a very simple 4 step workflow.
To begin the test, the user scans or enters sample information. The cartridge code is then scanned into the embedded Vivalytic software. The user then adds sample into the dedicated cartridge slot, closes the lid and inserts the cartridge into the Vivalytic.
The touchscreen display will countdown the time remaining to test completion. Results will be displayed on the screen. Multiple Vivalytics can be wirelessly connected allowing the user to control multiple tests at one time all reporting to a master Vivalytic platform.
Want to know more?
Contact us or visit our COVID-19 Monitoring & Management page
Related Products
Viral Respiratory Infection Array
Vivalytic Test Cartridges
Vivalytic
Vivalytic Test Menu
New 39-minute COVID test available on Randox-Bosch Vivalytic
02 October 2020
New 39-minute COVID test available on Randox-Bosch Vivalytic
- The world’s fastest PCR based SARS-CoV-2 test for the point of care delivers reliable results in 39 minutes.
- Has a sensitivity of 98 percent and a specificity of 100 percent.
- Simultaneous testing of five people with one cartridge by pooling will be available from early October.
- Work is in progress to further reduce time to result.
A rapid new coronavirus test, which provides results for Covid-19 in just 39 minutes, is now available on the Vivalytic, a point of care platform brought to market by Randox Laboratories and Bosch.
The test for detection of the SARS-CoV-2 pathogen, is currently the fastest PCR test (the gold standard of test methods) worldwide, and is predestined for decentralized use in mobile test centres at service stations or in airports, so that people who take the test can obtain a reliable result while at the testing site.
Available now in Europe, the CE-approved test, which has a sensitivity of 98 percent and a specificity of 100 percent, helps avoid time in quarantine, relieve laboratories, and make travel and work safer again.
“Rapid and accurate testing plays a crucial role in identifying cases of Covid-19 – to contain any outbreaks and limit the spread of the virus,” says Dr. Heather McMillan, Molecular R&D Manager at Randox Biosciences.
“This new rapid test will be a game-changer in the coronavirus testing landscape by allowing patients to receive their results at the point of care faster than ever before.”
Randox and Bosch launched the first rapid test for the Vivalytic analyser at the end of March, after just six weeks’ development.
As a multiplex test, it simultaneously checks samples for the SARS-CoV-2 virus as well as nine other respiratory diseases in two and a half hours, whereas the new accelerated test is exclusively for SARS-CoV-2.
“With our different coronavirus tests and variable analysis strategies, we open up a range of testing scenarios with a Vivalytic device – from screening all the way to supporting differential diagnosis for similar symptoms,” says Marc Meier, president of Bosch Healthcare Solutions GmbH.
And development work for Covid tests on the Vivalytic is ongoing: as of early October 2020, by pooling samples together it will be possible to simultaneously evaluate five samples in one test cartridge and at a comparable speed – a world first.
This will increase available testing capacity, by enabling fully automated processing of more than 160 samples a day using a Vivalytic device.
Key Benefits of SARS-CoV-2 test on Vivalytic point of care platform
The advantages of the rapid SARS-CoV-2 test on Vivalytic lie not only in speedy analysis, but also in ease of use. A sample is taken from the nose or throat using a swab, and placed in the test cartridge. Then the cartridge, which contains all the reagents required for the test, is inserted into the Vivalytic device for automated analysis.
- Turnaround time of 39 mins from sample entry to result.
- The SARS-CoV-2 rapid test has recently received CE marking.
- The SARS-CoV-2 pooling test can run up to 5 samples on-board one single cartridge.
- Easy 4-step user-friendly process from sample entry to result. Minimal training required.
- Detection from real-time PCR from Nasopharyngeal and/or Oropharyngeal swab.
- Suitable for use in any laboratory and non-laboratory settings.
The development of the new Vivalytic PCR singleplex test is part of a research and development project funded by the German Federal Ministry of Education and Research (BMBF).
For more information please contact marketing@randox.com
RESEARCH
BIOPHARMA
CLINICAL LAB
BIOREAGENTS
Vivalytic | Test Cartridges






Vivalytic | Cartridges
–
Powered by Biochip Technology
Simple & Accurate POC Molecular Diagnostics
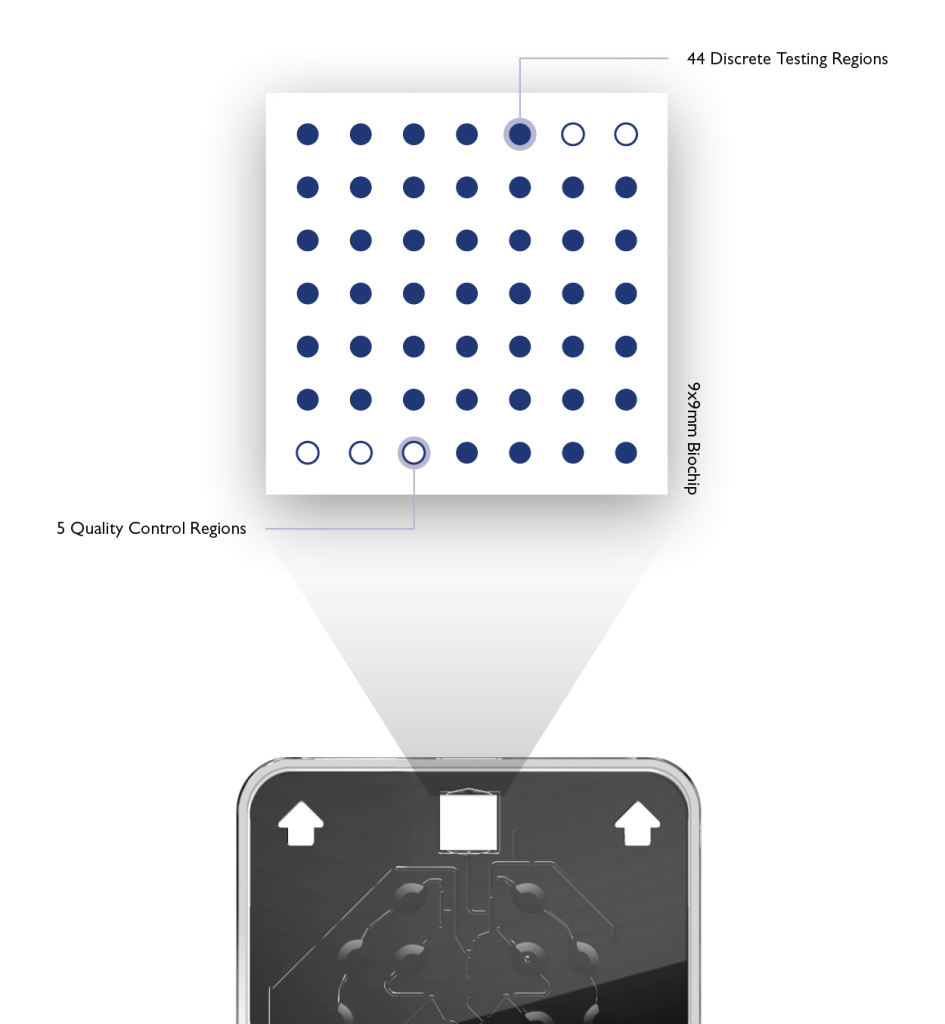
Vivalytic cartridges are compact, technologically advanced Molecular Diagnostic tests utilising micro-fluidics to enable simple and accurate diagnostic testing. Vivalytic cartridges are powered by a variety of technologies, dependent upon the test application. High-Plex and Low-Plex tests can be analysed on the Vivalytic. High-Plex tests utilise Randox patented Biochip Array Technology, enabling end-point qualitative PCR and providing multiple test results from each sample. Low-Plex tests are based on a variety of detection methods including real-time qualitative PCR and melting curve analysis.
Randox patented Biochip Technology allows simultaneous detection of multiple targets from a single patient sample. The biochip detection system is based on a chemiluminescent signal, this is the emission of light, without heat, as a result of a chemical reaction. Each biochip is prefabricated with spatially discrete testing regions (DTR’s).
Each DTR represents an individual test. Each DTR can be occupied with oligonucleotides specific to a pathogen or target of interest. The High-Plex capabilities of Biochip Technology eliminates the need to run multiple time consuming and sample intensive assays.
An enzyme is used to catalyse the chemical reaction of the biochip which generates the chemiluminescent signal. The light emitted from the chemiluminescent reaction that takes place in each DTR is simultaneously detected and quantified using a Charge – Coupled Device (CCD) Camera. This CCD Camera simultaneously records the light emission from all the DTRs on each biochip. The Vivalytic automatically generates a result report for all targets.

Want to know more?
Contact us or visit our COVID-19 Monitoring & Management page
Related Products














