Acute Kidney Injury and Antimicrobial Stewardship
Acute Kidney Injury and Antimicrobial Stewardship
An estimated 1 in 5 hospital admissions in the UK is associated with acute kidney injury1, providing a clear illustration of the need for novel, rapid detection methods. Our latest whitepaper looks at this common condition and the links between Acute Kidney Injury and Antimicrobial Stewardship. For more details on the things discussed in this article, you can download the full whitepaper below.
Acute Kidney Injury
Acute Kidney Injury is defined as a sudden loss of kidney function. This causes a disruption in the kidneys’ ability to filter waste out of your blood resulting in an accumulation of waste products as well as other imbalances.
The loss of kidney function is the result of a sudden reduction in glomerular filtration rate (GFR), the process through which waste is extracted from the blood and is often reversible2.
Aetiology of Acute Kidney Injury
The differential pressure existing between the glomerulus and Bowman’s is the driving mechanism for glomerular filtration2. This pressure contrast is influenced by the combined resistances of the afferent (leading to the glomerulus) and efferent (leading away from the glomerulus) vascular pathways in the kidney. Under normal kidney function, these resistances are in equilibrium, facilitating the proper functioning of the GFR. For example, an increase in efferent resistance restricts the blood flow out of the kidney, elevating pressure inside the kidney and reducing GFR, and vice versa2. However, in AKI, the decline in renal blood flow and GFR has a pathological origin. The pathophysiology of AKI can be classified as prerenal, intrinsic renal, or postrenal.
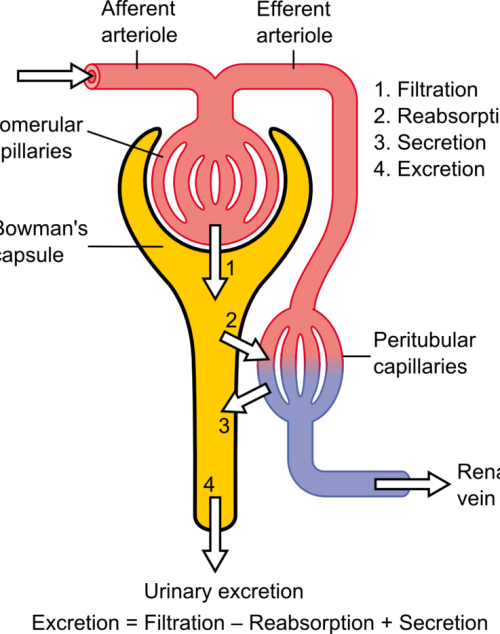
Pre-renal Acute Kidney Injury
Pre-renal AKI is caused by reduced afferent blood flow or, in other words, increased afferent resistance. While tubular and glomerular functions generally remain intact, pre-renal AKI may be caused by systemic hypoperfusion (decreased blood flow) or selective hypoperfusion to the kidney, caused by renal artery stenosis or aortic dissection3.
Intrinsic Renal Acute Kidney Injury
Renal AKI describes the conditions which affect the glomerulus or tubule, for example, acute tubular necrosis and acute interstitial nephritis. This collection of conditions is associated with vasoconstrictor expression in renal afferent pathways2.
Post-renal Acute Kidney Injury
Post-renal AKI usually results from an obstruction in the filtration system. Causes of obstruction include kidney stones, tumours, or blood clots, commonly in the bladder outlet. Obstruction affecting one side might not invariably lead to acute kidney injury, especially when the impediment develops slowly, such as with tumour growth. This is because the unaffected kidney might be able to adjust and make up for the compromised functionality3
Symptoms & Treatment
AKI often manifests with decreased urine output as its primary symptom. However, additional symptoms, when present, can encompass fatigue, nausea, vomiting, or confusion4. To achieve an accurate diagnosis, a comprehensive review of the patient’s medical history and a physical examination are essential to identify the underlying cause of the condition.
The treatment and management of AKI are contingent upon the root cause of the condition. In milder cases, measures are implemented to maintain appropriate levels of fluid, electrolytes, and blood pressure. Nutritional support may also be administered when necessary. In the most severe instances of AKI, dialysis may be warranted to compensate for the diminished kidney function5.
Creatinine serves as a valuable diagnostic tool for evaluating renal conditions, including kidney health, GFR, and muscular dystrophy. However, abnormal serum creatinine (SCr) levels only become evident when a significant portion of the renal mass is compromised. The kidneys possess an impressive capacity to adapt to reduced function, which means that a considerable loss of function or GFR is necessary to influence SCr levels. This poses a challenge when it comes to early detection of AKI6.
Novel biomarkers, KIM-1, NGAL, Clusterin, and Cystatin C, are associated with AKI2 and can be analysed through molecular testing. These new methods can provide a fast and accurate assessment of an individual’s kidney health, at a much earlier stage than SCr quantification2.
Antimicrobial Stewardship
Antimicrobial Stewardship (AMS) programs are specifically crafted to enhance the efficiency of antimicrobial utilization, curtail the emergence of Antimicrobiasl Resistance (AMR), and enhance patient outcomes7. These programs encompass a variety of approaches, such as educational initiatives, training, the establishment of guidelines and protocols, ongoing monitoring and feedback regarding antimicrobial usage, and the management of antimicrobial formularies. Through the promotion of prudent antibiotic utilization, AMS programs contribute to the safeguarding of the efficacy of currently available antimicrobial agents and the deceleration of AMR development7.
Antibiotics and Acute Kidney Injury
Various antibiotics are associated with the progression of AKI due to their nephrotoxicity which can cause severe damage to the kidneys. These antibiotics include polymyxins, aminoglycosides and the commonly used, vancomycin8.

Randox Renal Injury Detection
Using the patented Biochip Technology, the Randox Acute Kidney Injury (AKI) array, available on the Evidence Investigator, simultaneously tests for four novel biomarkers (KIM-1, NGAL, Clusterin, Cystatin C) delivering an early diagnosis and monitoring of treatment efficacy. Multiplex testing better captures reduced renal function, as each biomarker reflects different mechanisms that result in similar injury outputs, allowing for a more accurate picture of the underlying cause of AKI. Along with being able to identify AKI at a much earlier stage, this array provides an accurate and sensitive solution for the diagnosis and monitoring of AKI.
If you’d like some more information on the Randox Acute Kidney Injury Array or would like to add this technology to your laboratory, take a look at our website at https://www.randox.com/acute-kidney-injury/ or get in touch today at marketing@randox.com.

References
- NICE. How common is it? Acute Kidney Injury . Published July 2023. Accessed October 2, 2023. https://cks.nice.org.uk/topics/acute-kidney-injury/background-information/prevalence/
- Adiyanti SS, Loho T. Acute Kidney Injury (AKI) Biomarker.; 2012.
- Manzoor H, Bhatt H. Prerenal Kidney Failure.; 2023.
- NHS. Acute Kidney Injury. NHS. Published 2023. Accessed July 31, 2023. https://www.nhs.uk/conditions/acute-kidney-injury/
- Goyal A, Daneshpajouhnejad P, Hashmi M, Bashir K. Acute Kidney Injury . In: StatPearls [Internet]. StatPearls Publishing ; 2023.
- Rule AD, Lieske JC. The estimated glomerular filtration rate as a test for chronic kidney disease: Problems and solutions. Cleve Clin J Med. 2011;78(3):186-188. doi:10.3949/ccjm.78a.11004
- Baur D, Gladstone BP, Burkert F, et al. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17(9):990-1001. doi:10.1016/S1473-3099(17)30325-0
- Clifford KM, Selby AR, Reveles KR, et al. The Risk and Clinical Implications of Antibiotic-Associated Acute Kidney Injury: A Review of the Clinical Data for Agents with Signals from the Food and Drug Administration’s Adverse Event Reporting System (FAERS) Database. Antibiotics. 2022;11(10):1367. doi:10.3390/antibiotics11101367
National STIQ Day 2022
National STIQ Day 2022
14th January, National STIQ day was launched to get people thinking about the importance of sexual health and encourage everyone to get regular health checks.
More than 30 different bacteria, viruses and parasites are known to be transmitted through sexual contact. Eight of these pathogens are linked to the greatest incidence of sexually transmitted diseases (STI’s). STI’s are spread predominantly by sexual contact, including vaginal, anal and oral sex. Some STI’s can also be transmitted from mother-to-child during pregnancy, childbirth, and breastfeeding.
Any individual can catch an STI regardless of what age they are, their sexuality or how many sexual partners they have, as it only takes one sexual encounter to increase the risk of catching an infection. The importance of regular STI testing allows individuals to get peace of mind and take control of their sexual health as many infections don’t present symptoms and it’s advised that testing is required if individuals are concerned about their health, present symptoms or change sexual partner. Regular screening can aid in detecting an infection in the early stages and help to reduce the risk of such complications.
The increase in STI’s underline the need for urgent action which many reports have highlighted the need of ongoing inaction and development of strategies to improve sexual health. It has been widely noted by the CDC that over 20 million new STI’s are detected each year. (1) Public Health England also reported that 468,342 diagnoses of STIs had been reported in England in 2019 – a 5% increase from 2018 with a 26% increase in gonorrhoea infections, 10% increase in syphilis and 7% increase in the number of consultations at national sexual health services. It’s also widely noted that chlamydia testing, which is most common in young adults has declined by 13% since 2015 and 2% of all individuals tested had received a positive diagnosis. (2) (3)
Here at Randox, we offer solutions for clinical laboratories, point of care testing solutions and home STI testing kits for convenience and discretion. Randox provides the broadest STI testing menu on the market. Detecting 10 bacterial, viral, and protozoan infections, the STI test provides a comprehensive sexual health profile. The CE-marked STI Array provides the identification of co-infections, often in asymptomatic individuals and enable antibiotic stewardship.
The Randox test presents excellent precision, specificity, sensitivity and accuracy for STI diagnoses, which reduces the risk of false reporting and unnecessary confirmatory tests. The Randox simultaneous multiplex test means smaller sample volumes are required, enabling faster throughput and rapid patient diagnosis saving time and money for clinical and laboratories.
The Randox STI Multiplex test detects the following infections:
- Chlamydia trachomatis (CT)
- Neisseria gonorrhoeae (NG)
- Trichomonas vaginalis (TV)
- Mycoplasma genitalium (MG)
- Treponema pallidum (Syphilis) (TP)
- Herpes simplex virus 1 (HSV-1)
- Herpes simplex virus 2 (HSV-2)
- Haemophilus ducreyi (HD)
- Mycoplasma hominis (MH)
- Ureaplasma urealyticum (UU)
Does your laboratory or clinic carry out STI testing? Our molecular analyser, the Bosch Vivalytic and Evidence Investigator, powered by patented Biochip Array Technology, could be the diagnostic solution you need!
Solutions for the Laboratory
54 SAMPLES ● 1 TEST ● 10 INFECTIONS
The Evidence Investigator is a compact semi-automated benchtop analyser ideal for medium throughput laboratories.
- Sample Type: Swab or Urine
- Sample Volume: 300 μl
- Detection Method: Randox Biochip Technology (end-point PCR)
- Time to Result: 5 hours 30 minutes
STI Testing at the Point of Care
1 SAMPLE ● 1 TEST ● 10 INFECTIONS
The Bosch Vivalytic enables sample to answer, cartridge based molecular diagnostics at
the point of care. Powered by Randox Biochip Technology.
- Sample Type: Swab or Urine
- Sample Volume: 300 μl
- Detection Method: Randox Biochip Technology (end-point PCR)
- Time to Result: 2 hours 30 minutes
For more information about our STI Arrays or Vivalytic email: marketing@randox.com
- https://www.cdc.gov/std/life-stages-populations/adolescents-youngadults.htm
- https://www.tht.org.uk/news/rise-stis-underlines-need-urgent-action
- Sexually transmitted infections (STIs) (who.int)
Related Products and Services
Evidence Investigator
Biosciences
ANTIBODY TEST
Vivalytic
Why are Neutralising Antibody tests important now?
27 October 2021- Why are Neutralising Antibody tests important now?
Being prone to more frequent infections may mean that you have a weak immune system. With everything going on in the world, including COVID-19, this is important information that you need to know about yourself. As it stands, the pace of the vaccine booster rollout has slowed down and priority is given to people who are most vulnerable from developing a COVID-19 infection. This will offer those individuals the fullest protection against the virus this winter. According to John Roberts, from the COVID-19 Actuaries Response Group, “At the start of the booster campaign, the health secretary Sajid Javid said: ‘We will protect the most vulnerable through the winter months’. But at the current rate it is going to be well through winter before we get through those first groups.” This was stated on a BBC news article. (1)
This leads into the question that everybody is wondering…. Do we really need the booster vaccine? Infectious diseases expert, Professor Angus Dalgleish has commented during an interview on Good morning Britain that we should try another strategy. A strategy where we should test people’s immunity first to see if they need the booster vaccine. He stated, “It should not be difficult for one test to see what your immune response to your last vaccine is or if you have had very bad COVID, have you got a good immune response and do you need the booster”. (2)
Laith Jamal Abu-Raddad, an infectious-disease epidemiologist at Weill Cornell Medicine—Qatar in Doha has also stated in an article from Nature that “Wasting resources on boosters for those who are already protected against severe disease does not really make too much sense,” (3)
The Randox SARS-CoV-2 Neutralising Antibody tests detects antibody levels post-vaccination to determine eligibility for a booster vaccination. These tests utilise patented biochip technology to detect neutralising antibodies to the Wuhan and Delta SARS-CoV-2 variants. There is a need for tests of this kind to provide an accurate estimate of immunity, monitor vaccine effectiveness and the frequency of post-vaccine breakthrough infections with variants of concern. Recent studies indicate that the delta variant is capable of re-infection even in fully vaccinated individuals and that a significant proportion of fully vaccinated individuals with breakthrough infections can transmit the virus to others.
The SARS-CoV-2 Neutralising Antibody tests are a quick and effective way to determine:
- Longevity of immune response with response to post-vaccine infection, and variants of concern.
- Population surveillance and testing of those at risk of sub-optimal vaccine response.
- Measure antibody levels post-vaccination to determine eligibility for a booster vaccination.
- Accurately detects antibodies that are capable of inhibiting virus replication and neutralizing the infectivity of the virus.
Want to know more?
For more information on SARS-CoV-2 Neutralising Antibody tests please visit the link below,
SARS-CoV-2 Neutralising Antibody Test | Randox Laboratories
Visit booking.randox.ie to book your COVID-19 antibody test today!
Alternatively contact us via email: marketing@randox.com
Sources linked-
Related COVID-19 Products and Services
CERTIFLY LATERAL FLOW
HOME TEST - PCR
ANTIBODY TEST
LABORATORY TOOLS
SARS-CoV-2 Vascular & Multi-System Dysfunction Whitepaper





30 June 2021
SARS-CoV-2 Vascular & Multi-System Dysfunction Whitepaper Download
COVID-19, the disease caused by SARS-CoV-2, is an infectious disease caused by a newly discovered coronavirus. While many of whom become infected by the disease will experience mild to moderate cold or flu-like symptoms, those with health complications – such as autoimmune diseases, asthma, heart disease and diabetes – are at risk of developing serious illness and adverse outcomes.
As of September 2021, over 228 million COVID-19 cases have been confirmed worldwide, with an estimated one in six patients experiencing complications which could be life threatening, with over £116 billion spent by the UK government alone on measures to combat the disease. This drastic spending has been mirrored across the globe, with the significant economic burden expected to be suffered for generations to come.
The whitepaper provides a brief overview of the COVID-19 pandemic, before discussing vascular abnormalities and associated complications brought on by the virus, such as multi-system disfunction, acute respiratory disease syndrome (ARDS) and hepatic, renal & cardiovascular function.
Want to know more about Randox?
Contact us or visit our homepage to view more.
Our COVID-19 Products and Services
HOME TEST
ANTIBODY TEST
TRAVEL CERTIFICATE
LABORATORY TOOLS
Evidence Investigator: One Analyser for Multiple Food Testing Industries
28 September 2020
The Randox Evidence Investigator: One Analyser for Multiple Food Diagnostic Industries
The Randox Evidence Investigator has been validated for Randox Food Diagnostics across various food matrices including tissue, feed and cereals, honey, aquaculture, and milk, making it the perfect testing equipment solution for any food testing laboratory.
How can the Randox Evidence Investigator benefit me?
- The Randox Evidence Investigator is a multi-analyte quantitative drug residue screening analyser. Using Randox’s patented Biochip Technology, the analyser ensures screening food for drug residues is accurate and efficient, offering a range of laboratories comparable results to LC-MS/MS.
- Using multiplex technology, the Evidence Investigator can provide simultaneous detection for a wide range of analytes from a single sample, saving you time and resource, and getting the reliable results you need.
- The analyser uses unique image processing software to translate the Relative Light Units (RLU) generated from the chemiluminescent reactions into an analyte concentration.
- No manipulation of results is required, which reduces the scope for any operator error. The Randox Evidence Investigator provides excellent sensitivity with a quantitative concentration result (ppb) for each analyte tested.
- The analyser boasts an extensive test menu with tests for the most widely used drug residues and the most commonly detected mycotoxins in the feed production industry.
- When purchasing the Randox Evidence Investigator, you will receive the complete package required for sample analysis which includes the analyser, PC and imaging software, a thermoshaker and a barcode scanner.
Visit the Randox Food Diagnostics website for more information on this technology.
For all enquiries relating to food testing on any of our Randox analysers, please contact us via email at: info@randoxfooddiagnostics.com
Want to know more?
Contact us or visit our Randox Food Diagnostics website.
Related Products
Meat & Seafood
Milk
Honey
Wine
COVID-19 Cytokine Testing Solutions
COVID-19 Risk Stratification and Treatment Monitoring
Randox offer testing solutions for a comprehensive range of cytokines, cytokine receptors and growth factors designed to assist with COVID-19 risk stratification, monitoring of treatment efficacy and recovery. Utilising patented Biochip technology up to 12 cytokines and growth factors may be detected simultaneously from a single patient sample.
Cytokines play a vital role in the immune system and are known to be involved in the body’s response to a variety of inflammatory and infectious diseases. The over stimulation of these cytokines in response to infection is referred to as a ‘cytokine storm’ and strongly correlates with poor disease outcomes.
Cytokine storms are a common complication of SARS-CoV-2 (COVID-19) infection triggering viral sepsis, where viral replication and excessive, uncontrolled systemic inflammation may lead to pneumonitis, Acute Respiratory Distress Syndrome (ARDS), respiratory failure, shock, multiple organ failure, secondary bacterial pneumonia, and potentially death.


Cytokine Array I (12-plex)
Interleukin-1 (IL-1) is a regulatory and inflammatory cytokine, which exists in two forms, (IL-1α) and (IL-1β), which share 25% homology at amino acid level. IL-1α is produced as a biologically active 31 kDa precursor, which undergoes proteolytic cleavage yielding a 17 kDa protein of 159 amino acids.
There are two forms of IL-1, IL-1α and IL-1β ,which share 25% homology at amino acid level. IL-1β is synthesised as a biologically inactive precursor of 269 amino acids with a molecular mass of 31 kDa , which undergoes proteolytic cleavage by IL1 converting enzyme (ICE), which yields a 17kDa protein of 153 amino acids.
Interleukin-2 (IL-2) is an interleukin, a type of cytokine signaling molecule in the immune system. It is a 15 – 18 kDa protein which has varying degrees of glycosylation accounting for the observed molecular weight range. IL-2 regulates the activities of white blood cells (leukocytes, often lymphocytes) that are responsible for immunity.
IL-4 is a glycoprotein synthesised as a precursor protein of 153 amino acids. The first 24 amino acid residue signal peptide is cleaved to produce a 129 amino acid 15-19 kDa protein.
IL-6 is synthesised as a precursor protein of 212 amino acids. The N-terminal 28 amino acid residue signal peptide is cleaved to produce a 21kDa protein. It has two potential N-glycosylation sites which have no effect on bioactivity. Different post-translational alterations such as glycosylation and phosphorylation give various forms of IL-6 with molecular masses of 21.5-28 kDa. The IL-6 receptor is a strongly glycosylated 80 kDa protein of 449 amino acids. Two different forms of the receptor have been described that bind IL-6 with differing affinities, a soluble form of the IL-6 receptor has also been described. The IL-6 receptor is expressed on T cells, mitogen activated B cells, peripheral monocytes and some macrophage and B cell derived tumour cell types. IL-6 also influences antigen-specific immune responses and inflammatory reactions.
IL-8 is a member of a structurally similar family of cytokines called chemokines, which demonstrate chemotactic activity for neutrophils. IL-8 is a non-glycosylated protein of 8 kDa and consists of 99 amino acids with a 22 residue signal peptide that is cleaved to generate a 77 amino acid sequence. IL-8 is produced in response to proinflammatory stimuli. It is produced by monocytes, macrophages, fibroblasts, endothelial cells, keratinocytes, melanocytes, hepatocytes, chondrocytes, T-cells, neutrophils, and astrocytes.
Interleukin-10 (IL-10), alternatively known as B-cell-derived T-cell growth factor (B-TCGF), cytokine synthesis inhibitory factor (CSIF) or T-cell growth inhibitory factor is a homodimeric protein with a molecular weight of 18 kDa. It is produced as a 178 amino acid residue precursor, which is cleaved to give a mature protein of 160 amino acids. IL-10’s primary function is as an anti-inflammatory agent, which inhibits cytokine production by T cells and natural killer cells caused by activation of monocytes/macrophages.
IFN-γ is a cytokine critical to both innate and adaptive immunity, and functions as the primary activator of macrophages, in addition to stimulating natural killer cells and neutrophils. Biologically active interferon gamma is a 20 or 25 kDa glycoprotein depending on its glycosylation state. This lymphokine is synthesised as a 166 amino acid sequence but is cleaved to give a 143 amino acid residue.
Human EGF is produced as a long precursor protein of 1207 amino acids which is released by proteolytic cleavage to give a globular protein of 6.4 kDa consisting of 53 amino acids. EGF is a common mitogenic factor that stimulates the proliferation of different types of cells, especially fibroblasts and epithelial cells. EGF activates the EGF receptor (EGFR/ErbB), which initiates, in turn, intracellular signaling.
Monocyte chemoattractant protein (MCP-1) is part of the chemotactic family of cytokines called chemokines. ). It is a 76 amino acid peptide and has a molecular weight of 8.6 kDa. MCP-1 in particular chondrocytes confirming its role in inflammatory responses. MCP-1 has been implicated in a wide variety of inflammatory diseases such as artherosclerosis, delayed hypersensitivity reactions, rheumatoid arthritis, alveotitis and idiopathic pulmonary fibrosis.
Tumour necrosis factor alpha (TNFα) is a 157 amino acid 26 kDa transmembrane protein which is secreted as a soluble mature 233 amino acid homotrimer of 17 kDa by proteolytic cleavage. TNF-α is secreted by macrophages in response to stimuli for the induction of systemic inflammation. The binding of the ligand TNF-α to the TNF receptor (TNFR1) initiates the pro-inflammatory and pro-apoptotic signaling cascades.
Vascular endothelial growth factor (VEGF), also known as vascular permeability factor (VPF), is secreted as a glycosylated homodimeric protein of 46 kDa that is made up of two 24 kDa subunits linked by disulphide bonds. VEGF is expressed by vascularised tissue such as pituitary, brain, lungs, kidneys, heart and adrenal glands, although it is assumed that all tissues have the potential to produce the growth factor. VEGF is stimulated when cells become deficient in oxygen or glucose or under inflammatory conditions.
Ordering Information
| Cat. Number | Description | Kit Size |
|---|---|---|
| EV3508 | Cytokine Array I Evidence | 360 Biochips |
| EV3544 | Cytokine Array I Evidence | 180 Biochips |
| EV3513 | Cytokine Array I Evidence Investigator | 54 Biochips |
| EV3623 | Cytokine Array I High Sensitivity Evidence Investigator | 54 Biochips |
Cytokine Array III (4-plex)
Interleukin-5 (IL-5) is a disulphide linked homodimer and belongs to a family of structurally related proteins that includes: interleukin-2, interleukin-4, macrophage colony-stimulating factor, granulocyte macrophage colony-stimulating factor and growth hormone. It is a glycoprotein with the apparent molecular weight of recombinant IL-5 produced by mammalian cells in the range 45 to 60 kDa. The large variation in the molecular weight caused predominantly by the addition of heterogeneous carbohydrate chains.
Interleukin-15 (IL-15) is a 14 to 15 kDa protein of 114 amino acids. It contains 2 disulphide bonds and 2 N-linked glycosylation sites at the C-terminus1. IL-15 is expressed at the mRNA level in numerous normal human tissues in a broad range of cell types, including activated monocytes, dendritic cells, osteoclasts and fibroblasts. IL-15 has an essential role in natural killer (NK) cell development. It activates NK cell proliferation, cytotoxicity, and cytokine production and regulates NK cell/macrophage interaction. Studies have suggested that IL-15 may have a role in establishing innate immune responses and maintaining neutrophil-mediated inflammatory processes.
Granulocyte-macrophage colony stimulating factor (GMCSF) isolated from human sources is glycosylated with an apparent molecular mass of 23 kDa. The mature protein has 127 amino acids and is preceded by a hydrophobic leader sequence of 25 amino acids.
Macrophage inflammatory protein-1α (MIP-1α, CCL3) is a member of the CC chemokine subfamily whose members are known for chemotactic and proinflammatory effects and also for the promotion of homeostasis. MIP-1α is synthesised as a 92 amino acid precursor that is proteolytically processed to a mature protein of about 70 amino acids. MIP-1α has roles in inflammatory responses at sites of injury or infection by recruiting proinflammatory cells.
Ordering Information
| Cat. Number | Description | Kit Size |
|---|---|---|
| EV3680 | Cytokine Array III Evidence | 180 Biochips |
| EV3678 | Cytokine Array III Evidence Investigator | 54 Biochips |
Cytokine Array IV (5-plex)
Matrix metalloproteinase-9 (MMP-9) (gelatinase B) (92 kDa) is a member of the matrix metalloproteinase (MMP) family. MMP-9, one of the most widely investigated MMPs, regulates pathological remodeling processes that involve inflammation and fibrosis.
Soluble IL-2 receptor α (sIL-2Rα) results from the proteolytic cleavage of IL-2Rα at the cell surface by a membrane metalloproteinase; which is encoded by IL2RA on human chromosome. It’s widely noted in research that sIL-2Rα has been found in diseases caused by infections, autoimmune disease and organ transplantation.
Interleukin-6 (IL-6) is a multifunctional cytokine that regulates pleiotropic roles in immune regulation, inflammation, hematopoiesis, and oncogenesis. The IL-6 receptor complex belongs to the haematopoietic receptor superfamily and mediates the biological activities of IL-6. It consists of two distinct membrane bound glycoproteins, an 80 kDa cognate receptor subunit (IL-6R) and a 130 kDa signal-transducing element (gp130). The gp130 subunit is expressed in almost all organs including heart, kidney, spleen, liver, lung, placenta and brain.
Tumour necrosis factor receptor I is one of two specific, high affinity cell surface receptors that function as transducing elements, providing the intracellular signal for cell responses to tumour necrosis factor (TNF). TNF is a proinflammatory cytokine mainly produced by stimulated monocytes, macrophages and T-lymphocyte subsets. It has a key role in host defence and immunosurveillance, mediating complex cellular responses of a different, even contrasting nature. TNFRI has a molecular mass of 55 kDa1 and is expressed by almost all cell types2 especially those cells that are susceptible to the cytotoxic action of TNFI. TNFRs are detectable in normal serum, but their concentration increases significantly in inflammatory and non-inflammatory diseases.
Tumour necrosis factor receptor II (TNFRII) is one of two specific, high affinity cell surface receptors that function as transducing elements, providing the intracellular signal for cell responses to tumour necrosis factor (TNF). TNF is a proinflammatory cytokine mainly produced by stimulated monocytes, macrophages and T-lymphocyte subsets. It has a key role in host defence and immunosurveillance, mediating complex cellular responses of a different, even contrasting nature. TNFRII has a molecular mass of 75 kDa1. Although TNFRII is expressed by almost all cell types, it is expressed primarily by cells of the immune system, cells of myeloid origin and endothelial cells.
Ordering Information
| Cat. Number | Description | Kit Size |
|---|---|---|
| EV3659 | Cytokine Array IV Evidence | 180 Biochips |
| EV3661 | Cytokine Array IV Evidence Investigator | 54 Biochips |
Cytokine Array V (5-plex)
Interleukin-3 (IL-3) possesses diverse biological activities and was discovered independently in studies on its biological activities. IL-3 is a heavily glycosylated protein with a polypeptide chain of 133 amino acids. It occurs naturally in a diversity of glycoforms generated by the addition of carbohydrate groups which results in size heterogeneity from 28 to 45 kDa. The function of the extensive carbohydrate modifications of the IL-3 polypeptide is not known however IL-3 has been linked with various diseases including colorectal and pancreatic cancers.
Interleukin-7 (IL-7) is classified as a type 1 short-chain cytokine of the haematopoietin family, a group that also includes IL-2, IL-3, IL-4, IL-5, GM-CSF, IL-9, IL-13, IL-15, M-CSF, and stem cell factor. The human gene for IL-7 is located on chromosome 8q12-13. The amino acid sequence of IL-7 predicts a molecular weight of 17.4 kDa, but glycosylation results in an active protein of 25 kDa. IL-7 appears to be involved in the development of an effective immune system and also in the generation and maintenance of strong and effective cellular immune responses directed against cancer cells, or infectious diseases.
Interleukin-12 (IL-12) is a 75 kDa heterodimeric glycoprotein cytokine composed of disulphide linked p40 (40 kDa) and p35 (35 kDa) subunits that are derived from separate genes1. p35 is expressed in a limiting and tightly regulated fashion by many different cell types, however the expression of p40, though in greater quantities than required for p70 formation, appears to be restricted to antigen presenting cells. IL-12 stimulates IFN production, which is essential in resistance to intracellular protozoan, fungal and bacterial infections and, in addition, tumours. Traditionally, IL-12 is accepted as an important mediator of autoimmunity and is involved in a number of autoimmune diseases including rheumatoid arthritis, psoriasis, inflammatory bowel disease and insulin-dependent diabetes mellitus.
Interleukin-13 (IL-13) is a 12 kDa protein that folds into four I-helical bundles. It contains four potential N-glycosylation sites and four cysteine residues that form two intramolecular disulphide bonds. IL-13 shares a number of structural features and functional characteristics with IL-4. The IL-13 protein is approximately 25% homologous1 with IL-4 and belongs to the same I-helix protein family. IL-13 plays a dominant role in resistance to most gastrointestinal nematodes and also modulates resistance to intracellular organisms by regulating cell mediated immunity. IL-13 is the central mediator of allergic asthma, where it regulates eosinophilic inflammation, mucus secretion, and airway hyperresponsiveness. Although IL-13 is associated primarily with the induction of airway disease, it also has anti-inflammatory properties.
Interleukin 23 (IL-23) is member of the IL-12 family. The IL-12 family consists of cytokines IL-12(p40p35), IL-23(p40p19) and IL-27(EBI13p28), and monomeric and homodimeric p401. IL-23 is a heterodimeric cytokine composed of disulphide linked p19 and p40 subunits. IL-23 plays a role in a signaling pathway that triggers inflammation.
Ordering Information
| Cat. Number | Description | Kit Size |
|---|---|---|
| EV3666 | Cytokine Array V Evidence Investigator | 54 Biochips |
Immunoassay Platforms
Want to know more?
Contact us or visit our COVID-19 Monitoring & Management page
Related Products
Cytokine Array I Control
Cytokine Array I High Sensitivity Control
Cytokine Array III Control
Cytokine Array IV Control
COVID-19 Management of Kidney Injured Patients – CKD & AKI
COVID-19 Management of Kidney Injured Patients
Analysis of COVID-19 patients revealed that Acute Kidney Injury (AKI) is common and associated with a very high mortality rate highlighting the need for more accurate patient testing. Further to this the National Institute for Health and Care Excellence (NICE) recommend that all COVID-19 patients are assessed for AKI on admission to hospital and their condition monitored throughout their stay. The complications with serum creatinine measurement alone for the detection of impaired kidney function are well known. To address this issue, Randox have developed three multi marker kidney function arrays for early detection of renal impairment. Individuals with pre-existing kidney injury are at an increased risk of COVID-19, those with severe CKD (stages 3-5) are at a higher risk of complications.
Utilising patented Biochip Technology, the Randox Chronic Kidney Disease (CKD) and Acute Kidney Injury (AKI) arrays could improve COVID-19 risk stratification whilst monitoring the effectiveness of treatment.

Randox Chronic Kidney Disease (CKD) Array I (7-plex)
EGF regulates renal cell proliferation, fibrosis and inflammation and is produced in response to renal injury.
IL-8 endothelial-derived chemokine involved in recruiting neutrophils to sites of injury and stimulating their response.
sTNFR1 is used to identify an increase in inflammatory conditions such as CKD.
FABP1 binds long-chain fatty acids, contributing to reducing oxidative stress in the kidneys.
sTNFR2 is used to identify an increase in inflammatory conditions such as CKD.
D-Dimer is a fibrin degradation product, and an index of both coagulation and fibrinolysis.
MIP-1 alpha plays a roles in inflammatory responses at sites of injury or infection.
Randox Chronic Kidney Disease (CKD) Array II (4-plex)
CRP is an acute phase reactant involved in inflammation.
Cystatin C is well recognised marker of kidney filtration dysfunction and injury.
C3a des Arg is a representative of complement component C3a which produces local inflammatory responses.
NGAL is among the current state-of-the-art in CKD biomarkers.
Randox Acute Kidney Injury (AKI) Array (4-plex)
This marker is highly upregulated in kidney tubule cells following nephrotoxic injury severe enough to result in acute renal failure, acute tubular necrosis or acute tubulo-interstitial nephropathy.
Due to its small size and basic pH, Cystatin C is freely filtered by the glomerulus. It is then reabsorbed by tubular epithelial cells and subsequently metabolized. Accumulation of Cystatin C in urine is specific for tubular kidney damage and suggests reduced reabsorption at the proximal tubules as a result of toxicant-induced kidney injury.
Expression of Clusterin is upregulated following a variety of renal injuries and is detectable in urine following acute kidney injury induced by administration of nephrotoxic agents. This occurs before the profound renal transformations that give rise to changes in creatinine and BUN.
KIM-1 is a 30kDa type 1 transmembrane glycoprotein found on actvated CD4+ T cells. It is undetectable in healthy kidney tissue but is expressed at very high levels in proximal tubule epithelial cells in the kidney after toxic injury.
The Evidence Investigator
Meet the Evidence Investigator
The Randox CKD & AKI arrays have both been developed for the Evidence Investigator, a semi-automated benchtop immunoassay analyser.
The CKD & AKI array’s would improve COVID-19 risk stratification whilst monitoring the effectiveness of treatments by simultaneously and quantitatively detecting multiple serum biomarkers of kidney damage-related analytes from a single sample.




Want to know more?
Contact us or visit our Investigator Webpage
Alzheimer’s Disease Array
Rapid Identification of Alzheimer's Disease Risk
The Randox ApoE4 Array is a rapid and highly sensitive blood test facilitating direct ApoE4 genotyping without the need for molecular genotyping. ApoE exists as three common isoforms; ApoE2, ApoE3 and ApoE4. As such, six common ApoE genotypes exist in the general population. Alzheimer’s Disease risk is significantly increased in individuals with the ApoE4 allele. The below table provides an indication of risk associated with the six common ApoE genotypes.




Biomarkers Tested
ApoE is a major cholesterol carrier, responsible for lipid homeostasis by mediating lipid transport from one tissue or cell type to another. In the central nervous system, ApoE is mainly produced by astrocytes, and transports cholesterol to neurons via ApoE receptors. ApoE exists as three common isoforms; ApoE2, ApoE3 and ApoE4.
ApoE4 is established as the strongest genetic risk factor for Alzheimer’s Disease. ApoE4 triggers inflammatory cascades that cause neurovascular dysfunction, including blood-brain barrier breakdown, leakage of blood-derived toxic proteins into the brain and reduction in the length of small vessels.
Apo E4 is one of three common isoforms of Apo E and is recognised as a major genetic risk factor the development of Alzheimer’s disease. Apo E4 triggers inflammatory cascades that cause neurovascular dysfunction, including blood-brain barrier breakdown, leakage of blood-derived toxic proteins into the brain and reduction in the length of small vessels.
The Evidence Investigator
Meet the Evidence Investigator
The Randox ApoE4 Array (EV4113) is a research use-only product developed for the Evidence Investigator, a semi-automated benchtop immunoassay analyser.
The ApoE4 Array (EV4113) simultaneously measures both total ApoE and ApoE4 protein levels directly from a plasma sample. The Apo E4/total ApoE ratio can classify the ApoE4 status of the plasma sample as negative or positive. Furthermore, the Array has demonstrated potential to classify ApoE4 positive plasma samples as being derived from a heterozygous (one copy of ApoE4 gene) or a homozygous (two copies of ApoE4 gene) individual. Therefore, risk for the development of Alzheimer’s Disease can be assessed.




Publications
Want to know more?
Contact us or visit our Cerebral Array webpage.
Hospitals in Wuhan and Guangzhou roll out new coronavirus test developed by Randox scientists
02 March 2020
Hospitals in Wuhan and Guangzhou roll out new coronavirus test developed by Randox scientists
Randox’s pioneering new test for coronavirus, which identifies COVID-19 and differentiates it from nine other respiratory infections, is to be used in Chinese hospitals.
The comprehensive test, which is being shipped this week to hospitals in Wuhan and Guangzhou, has been developed on Randox’s unique technology platform, the Biochip. This allows for each patient to be simultaneously tested for a range of respiratory infections inclusive of all known coronaviruses.
The Coronavirus Biochip tests each single patient sample for the SARS-CoV-2 virus that causes COVID-19, as well as nine other respiratory viruses, including SARS, MERS, and Influenza A and B. This enables clinicians to quickly and efficiently differentiate between potentially lethal and non-lethal infections, prioritise patients and administer appropriate and timely treatment.
Dr Peter FitzGerald, Managing Director of Randox Laboratories, commented;
“Current technologies being used to diagnose COVID-19 are focused on simply detecting the presence or lack of this singular strain and therefore neglect to differentiate it from other respiratory infections. At Randox we have developed a multiplex Viral Respiratory Infection Array that tests for COVID-19 and nine other infections simultaneously, and are delighted that this new technology will be deployed in Wuhan and other cities across China.
“The Coronavirus Biochip will eliminate the need for multiple back-and-forth tests before the root cause of symptoms is found, thereby empowering clinicians to make faster and better-informed decisions.”
Due to be used in hospitals in Wuhan, the epicentre of the coronavirus outbreak, as well as in The First Affiliated Hospital of Guangzhou Medical University, the new testing technology is capable of processing 324 patient samples, generating 3240 reportable results, in just 8 hours.
The panel includes two tests for COVID-19, a specific test (SARS-CoV-2) and a confirmatory test (Sarbecoviris) on the same panel, as recommended by the World Health Organisation. This avoids the need to repeat the test, and importantly, reduces the likelihood of incorrect diagnosis, ensuring appropriate containment and reducing the risk of further contamination. This faster and more comprehensive testing will ultimately support the health service in China by facilitating the efficient use of valuable healthcare resources.
Byron Wang, CEO of Beijing Promed, Randox’s partner in providing the new coronavirus test in China, commented;
“We welcome the support of the global community in assisting us combat COVID-19 at this time. Randox is a highly regarded In Vitro Diagnostic company in China and has supported our market with high quality products for many years. We look forward to supplying this test to hospitals located within the area of greatest need and believe it will make a real difference.”
Dr FitzGerald concluded;
“Randox is committed to saving and improving lives on a global scale and we know that this new COVID-19 test will make a significant contribution to the global coronavirus containment effort.”
The Coronavirus Biochip tests simultaneously for Coronavirus SARS-CoV-2 (COVID-19), Sarbecoviris (SARS, SARS like, COVID-19), Coronavirus 229E/NL63, Coronavirus OC43/HKUI, Middle East Respiratory Syndrome Coronavirus (MERS-CoV), Adenovirus A/B/C/D/E, Enterovirus A/B/C, Influenza A, Influenza B and Rhinovirus A/B.
The Randox Coronavirus Biochip complies with guidelines from the Centres for Disease Control and Prevention and the World Health Organisation.
For more information or to arrange interviews, please contact the Randox PR team on 028 9442 2413 or email randoxpr@randox.com
QNOSTICS
QCMD
BIOSCIENCES
Researching the Evidence Investigator
The Evidence Investigator analyser is based on the award-winning Biochip Array Technology (BAT). Biochip Array Technology is a multi-analyte testing platform allowing the simultaneous quantitative or qualitative detection of a wide range of analytes from a single sample.
The Evidence Investigator is a semi-automated benchtop analyser which is tailored for the areas of research, forensic, clinical, molecular and veterinary testing. The key feature is the fast turn-around time the Evidence Investigator can process up to 44 results from a single sample, with a maximum throughput of up to 2375 tests per hour. The Evidence Investigator saves time because it will carry out multiplex testing which will allow multiple tests to be carried out from a single patient sample which in return will save time and resources. The machine is more suitable for medium throughput laboratories.
The technology offers a wide-ranging and diverse test menu which will benefit the research areas immunology, metabolic, Sport & Exercise, oncology and cardiovascular. Randox Research provides the Evidence Investigator to the five research areas to help their research become more efficient, cost effective and accurate.
Randox works extremely hard with their research and development, over 16% of turnover is reinvested in R&D. The current collaboration Randox works along with are Royal Victoria Hospital, Queen’s University Belfast and Cambridge University.
Randox Biosciences are dedicated to assisting research projects to completion and will make available the technology to ensure the universities receive highly accurate results for their research. Randox creates their products in-house therefore provides the flexibility of the research project. Randox can provide the university a full range of arrays, biomarkers and analysers to meet their requirements of the research.
Within Cardiovascular Research, Randox offer a comprehensive menu of cytokines. The combination of highly specific antibodies and advanced chemistries enables cytokines, cytokine receptors and growth factors to be detected simultaneously in a single sample, providing valuable information relating to each cytokine under test and possible associations between cytokines in each sample which will benefit the research. Randox offer excellent tools for universities and hospitals researchers such as routine and novel assays and can provide research analysers such as the Evidence Investigator which is suitable for medium sized laboratory.
Oncology Research has 20 biomarkers that can be custom-made to be used on the Biochip. Randox Biosciences offers a wide and extensive test menu to researchers to enable the specific product tailored to meet their clinical trail requirements.
Metabolic & Nutrition Research is another area Randox offers a wide-ranging range of tests specifically directed to Metabolic and Nutrition Research. Randox offers reagents and arrays on the award-winning Biochip Array Technology.
For more information on our Research areas and the tests that we can provide, contact us at – Info@RandoxBiosciences.com

















