Zachowanie precyzji jest kluczem do podejmowanie decyzji diagnostycznych

Randox Laboratories oferuje kompleksowe rozwiązania w zakresie testów lipidowych i kardiologicznych. Wysokiej wydajności odczynniki do wykrywania standardowych czynników ryzyka i odczynniki wskazujące biomarkery, powiązane z podwyższonym ryzykiem w połączeniu z ofertą kontroli jakości obejmującą szeroki zakres markerów kardiologicznych oraz lipidów na istotnym klinicznie poziomie, co tworzy zróżnicowany produkt, który spełnia wymagania każdego laboratorium.
Odczynniki firmy Randox do testów kardiologicznych i lipidowych
W obliczu potrzeby prowadzenia bardziej rozbudowanej analizy profilu lipidowego, w celu dokładniejszej oceny ryzyka zapadalności na choroby układu krążenia, zarówno w przypadku najczęściej ocenianych jak i mniej popularnych czynników ryzyka, pojawiło się zapotrzebowanie na narzędzia umożliwiające identyfikację oraz redukcję ryzyka. Firma Randox oferuje złożony produkt, którego profil kardiologiczny obejmuje zarówno wysokoprzepustowe odczynniki służące detekcji najczęściej testowanych czynników ryzyka jak i odczynniki do badania biomarkerów związanych z oceną późniejszej zapadalności na chorobę.
Dostępne odczynniki
| Monitorowanie oceny ryzyka | |
| Cholesterol HDL | Apolipoproteina C-II |
| Cholesterol LDL | Apolipoproteina C-III |
| Cholesterol całkowity | Apolipoproteina E |
| Trójglicerydy | HDL3-C |
| Niskocząsteczkowy cholesterol sLDL | sPLA2-IIA |
| Lipoproteina (a) | Homocysteina |
| Apolipoproteina A-I | Wysokoczuły CRP |
| Apolipoproteina B | Adiponektyna |
| Apolipoproteina A-II | |
| Diagnoza zawału mieśnia sercowego | |
| Typ sercowy białka wiążącego kwasy tłuszczowe (H-FABP) | Mioglobina |
| CK-MB | |
| Monitorowanie terapii | |
| Digoksyna | TxBCardio™ |
W celu uzyskania dalszych informacji na temat zakresu testów odczynników kardiologicznych zapraszamy na stronę www.randox.com/cardiology-reagents/ lub prosimy o kontakt pod adresem reagents@randox.com
Kontrola jakości Acusera do lipidów
Kontrola jakości Acusera do lipidów jest wytwarzana w 100% z ludzkiej surowicy w celu zapewnienia, najbardziej zbliżonego materiału do próbek pacjentów. Jak wszystkie nasze kontrole lipidowe są liofilizowane, nie zawierają one żadnych stabilizatorów ani konserwantów, które mogą mieć wpływ na ogólną wydajność kontroli. Kontrole Lipidowe, wieloparameterowe, odpowiednio skonsolidowane mają czteroletni okres trwałości materiału od daty produkcji co gwarantuje optymalizację kosztów w laboratorium.
W celu uzyskania dalszych informacji na temat zakresu testów kontroli lipidowych zapraszamy na stronę www.randox.com/lipid-quality-controls/ lub prosimy o kontakt pod adresem acusera@randox.com
Płynna kontrola lipidowa Acusera
Złożona kontrola zaprojektowana do stosowania w rutynowym monitorowaniu dokładności i precyzji. Wartości mianowane i zakresy przypisane są do poszczególnych analizatorów (biochemicznych, immunologicznych i POCT) dla 8 markerów kardiologicznych.
Wygodna forma płynna kontroli, gotowa do użycia od razu skraca czas przygotowywania a stabilność próbki 30 dni po otwarciu dla wszystkich parametrów minimalizuje ilość odpadów oraz redukuje koszty. Dostępne są 3 poziomy kontroli pokrywające cały zakres badań, co pozwala ma wiarygodną ocenę wydajności oraz podejmowanie właściwych decyzji.
W celu uzyskania dalszych informacji na temat zakresu testów kontroli lipidowych zapraszamy na stronę http://www.randox.com/liquid-cardiac-quality-control/ lub prosimy o kontakt pod adresem acusera@randox.com
RIQAS Frequently Asked Questions (FAQ’s)

- How do I register for RIQAS?
Complete the RIQAS method questionnaire and enrolment document for the programmes you wish to participate in. RIQAS will then issue you with a unique laboratory reference number. The enrolment document should be returned to RIQAS before the start of the cycle. These documents can be easily downloaded from the RIQAS website. Simply click on the programme of interest and download the relevant documentation.
- What if my current method is not listed in the method questionnaire or enrolment document?
Use the method questionnaire to help you complete the registration of methods section on the enrolment document. If a code is not available for your method/assay please state the details of your method clearly in the appropriate section at the end of the enrolment document.
- How do I enter my EQA results?
Participants may conveniently enter their results online via RIQAS.Net. Alternatively results can be entered via the manual return sheet and submitted by fax or post before the final submission deadline.
- How do I know when to submit my EQA results?
Each RIQAS pack will contain a multi-lingual product insert containing instructions for use. The product insert also highlights the recommended date of analysis and more importantly the final date by which the results must reach Randox. The final date for submission of results can also be found on the RIQAS calendar. All results should reach RIQAS before 5pm GMT on the final submission date.
- How and when are RIQAS reports issued?
For most programmes reports are available within 72 hours of the final submission date (for RIQAS Serology Programmes, reports are sent via email within 7-10 days of the final submission date). The reports may be accessed online via RIQAS.Net or alternatively may be sent by email or post. Individual reports may be emailed to up to three addresses.
- Can I register multiple instruments for a single EQA programme?
Yes, laboratories can register up to five instruments per programme at no extra cost. Individual reports for each instrument plus a unique multi-instrument report are provided. The multi-instrument report allows for comparative performance assessment of each instrument. Additional sample packs may be ordered as required.
- What is the summary CSV file?
Laboratories can register to receive a CSV file containing a summary of their report statistics, acceptable limits and performance indicators for every sample. The file mirrors the information found on the quantitative report summary page but will also include the calculated SD and SDPA. If you wish to receive a summary CSV file, please indicate this by ticking the box on the enrolment document and include the email addresses to which the reports should be sent.
- Is RIQAS accredited to ISO/IEC 17043:2010?
Yes, in 2012 RIQAS celebrated gaining accreditation to ISO/IEC 17043:2010. This standard outlines general requirements for proficiency testing and demonstrates our commitment to quality whilst providing both participants and accrediting bodies with confidence in the schemes operation. Our accreditation to ISO/IEC 17043:2010 highlights the superior quality and excellence of RIQAS. **Please be aware that not all RIQAS programmes are accredited. Programmes marked with a “+” highlight the programmes not accredited.**
9. What if I don’t need all the parameters in a particular EQA programme?
Reduced parameter options are available for selected EQA programmes offering greater flexibility, whilst ensuring suitability for laboratories of all sizes and budgets.
10. How does the group reporting facility work?
The Group Reporting facility enables group co-ordinators to monitor the performance of satellite sites. Each individual laboratory in the group will receive an individual report, the group supervisor will receive a unique instrument group report comparing each laboratory’s performance within the group.
11. Will I receive a certification of participation?
Yes, RIQAS provides certificates as proof of EQA participation and performance for laboratory accreditation purposes. A complimentary certificate of participation for each RIQAS programme is available to participants at the end of each cycle, provided at least 50% of results have been returned. A certificate of performance is also supplied with the end-of-cycle report. Certificates will specify the cycle number, programme name and the LABORATORY / HOSPITAL NAME specified in the enrolment document.
12. Can you offer technical support and advice?
Unrivalled technical support is available through our team of RIQAS scientists and experts who are on hand to offer advice and to help you troubleshoot technical issues relating to our RIQAS programmes.
13. I have found a transcription error on my report. Can I submit the revised result?
Participants are permitted to submit corrected results up to 4 weeks after the final date of the sample. Although a new report will not be issued, results can be viewed on the charts of subsequent reports, showing “C” in place of the sample number. If a result is corrected and resubmitted to RIQAS before the final date for the current sample, it will be entered as a current result.
14. How do I notify a change of method if the cycle is already underway?
It is possible to change your units, method, instrument or reagent classification during a cycle. For participants using RIQAS.Net changes can be made in the method changes section of the data entry menu. Each RIQAS return sheet also has a section for method changes.
15. How do I add extra parameters to my registration?
Extra parameters can be added to a registration via RIQAS.Net using the method changes section on the data entry menu. A list of your registered laboratory reference numbers will appear on screen. Select the laboratory reference number for which you would like to add the assay details and select ‘Add Parameter’. A list of parameters you are not registered for will appear. Select the parameters you wish to add and complete the assay details. Parameters cannot be deleted on RIQAS.Net. If you wish to delete a parameter please contact RIQAS directly on mail@riqas.com.
A week dedicated to unsung heroes! – Medical Laboratory Professionals Week 2017
From April 23rd to April 29th we are celebrating Medical Laboratory Professionals Week! This is a week dedicated to raising awareness for those who work in a laboratory & the hard work that goes unnoticed every day in laboratories around the world.
Have you ever wondered what happens between submitting your patient sample and receiving your results? Have you ever wondered who conducts the detailed laboratory testing for your annual check-up such as cholesterol and glucose levels? Or who analyses these results? The answer, a Medical Laboratory Professional (MLP). MLP’s provide up to 70% of the medical laboratory results for physicians and others to make informed decisions about a patient’s diagnosis and aftercare treatment plan. The work that laboratory professionals do each and every day is integral to providing excellent patient care. They perform and interpret billions of laboratory tests every year.
Providing accurate and reliable test results is of the utmost importance for laboratory professionals and also for us at Randox. With a passion for Quality Control, and with more than 30 years’ experience developing Laboratory QC for the in vitro diagnostics market, we believe in producing high quality material designed to streamline procedures, whilst reducing costs in laboratories of all sizes and budgets. These qualities have been reflected in our Acusera true third party quality controls, Acusera 24.7 interlaboratory data management software, Acusera Verify Calibration Verification material and RIQAS, the largest international EQA scheme.
Randox Quality Control would like to take this opportunity to thank all the laboratory professionals around the world and especially our own laboratory staff – you truly are the “Unsung Heroes of Healthcare”.

QC Material Stability – Dig a Little Deeper
QC Material Stability
Stability has a number of different definitions, however, the most relevant to clinical diagnostics, and indeed quality control sera, is the “resistance to chemical change or physical disintegration”. Much like a chain, your quality control system is only as strong as its weakest link, or in this case analyte.
Whilst we appear to be stating the obvious here, this might not be as straightforward as it first appears. The product literature you peruse will help you decide what control best suits your needs, whilst many companies will state their control stability in the literature there are some instances where all may not be as it first appears. It is also important to note that some manufacturers may not make stability claims for some of the analytes listed in their control material. In such instances, you are required to validate these in-house, taking up precious time and resources.
Dig a Little Deeper
Whilst we understand that some analytes do have limitations due to their inherent nature, misleading analyte claims can cost the laboratory both time and money. In a recent survey conducted by Randox, 65.5% of respondents indicated that they felt stability was a ‘Very Important’ QC feature. As such it’s important that you look beyond the sales literature when it comes to control stability. Look out for exceptions in the small print of the control kit inserts. For example, if a control has a stability claim of 7 days at 2-8oC and a routine analyte like Cholesterol has a stability claim of just 2 days at 2-8oC then the true stability of the control is only 2 days. In such instances, there is a lot of potential for waste, as laboratories will be required to prepare a new vial of QC material every 2 days leading to increased costs and time. However, if you dig a little deeper into the controls and always read the small print, you could avoid such issues.
How can Randox Acusera benefit you?
For more than 30 years Randox has been shaping the future of clinical diagnostics with our pioneering high quality, cost effective laboratory solutions. Quality Control is our passion, we believe in producing high-quality material that can help streamline procedures, whilst saving money for laboratories of all sizes and budgets. We pride ourselves in not misleading our customers with false stability claims for our controls. With controls such as our Liquid Cardiac and Specific Proteins Controls, you could benefit from a 30-day open vial stability for all analytes, without exception.
By employing our Randox Acusera quality control materials you could benefit from;
Commutable controls, ensuring a matrix that reacts to the test system in the same manner as a patient sample, enabling an accurate and reliable assessment of instrument performance.
Accurate target values that won’t shift throughout the shelf life of the controls, eliminating the need to spend valuable time and money assigning values in-house.
Consolidation of test menu with controls comprising up to 100 analytes, reducing preparation time and storage space required.
Analytes present at clinically relevant levels ensuring accurate test system performance across the clinical range, maximising laboratory efficiency by eliminating the need to purchase additional high or low-level controls at extra expense.
True third party controls designed to provide an unbiased assessment of performance, our Acusera controls have not been manufactured in line with or optimised for use with any particular reagent, method or instrument.
For more information on any of our products, or to request a consultation from one of our QC Consultants, contact us via acusera@randox.com.
Randox celebrates British Science Week 2017
Randox celebrates British Science Week 2017
Last week was British Science Week 2017, an annual campaign that aims to inspire innovation and celebrate science. To mark the occasion, Randox Laboratories got involved by celebrating the innovation of each Randox product group. The product groups within Randox shared a series of posts, videos and blogs showcasing the #ScienceBehindRandox throughout British Science Week.

Randox
To initiate the Randox British Science Week campaign, Randox shared this video, which highlights the company’s dedication to improving health worldwide. The video provides an introduction about each product group, however throughout British Science Week, each product group has gone into further detail about the #ScienceBehindRandox.
Randox Careers, the RX series, Randox Reagents, Randox Quality Control, Randox Toxicology, Randox Biosciences, Randox Testing Services, & Randox Food Diagnostics all got involved in the British Science Week Campaign. You can read a snippet of each product groups post below, with videos and links to the full content also provided. We hope you enjoy learning about the #ScienceBehindRandox.
Randox – Dedicated to improving health wordwide.
Randox Careers
Joanne Darragh spent some time with Randox Careers to discuss her role as R&D Toxicology Manager.
“Working in this area has been both challenging and exciting as we are at the cutting edge of assay development. We work in a great team and we work along very closely alongside other departments such as Marketing & Sales so that we are in close contact with what the customer needs, which means we are producing relevant tests very quickly and effectively. Every day brings a new challenge.”
– Joanne Darragh, R&D Toxicology Manager
Listen to what Joanne had to say on the video above
Randox RX series
As part of British Science Week, the RX series caught up with Daniel Melly, one of our very talented Mechanical Design Engineers based in Randox Teoranta in Dungloe, Ireland.
Daniel was an integral part of the team involved in the design of our new semi-automated analyser, the RX misano. The RX series asked Daniel a few questions about why Randox created this analyser, the design process involved in creating such a unique system, and what his favourite features are.
“Randox set out in creating the RX misano with the philosophy of supplying the customer with a more modern, reliable, and aesthetically pleasing analyser than those that are currently available on the market. Robust part selection was always at the fore of any design decisions, and we feel that we have delivered on all of these requirements.”
– Daniel Melly, Randox Mechanical Design Engineer

Read the full interview the RX series had with Daniel here
The RX misano is currently unavailable to purchase in Germany
Randox Reagents
One unique test by Randox, adiponectin, is becoming an increasingly significant biomarker for health professionals. Low levels have been linked with several illnesses including metabolic syndrome, cancer and cardiovascular disease.
What is adiponectin?
Adiponectin is a protein hormone produced and secreted by fat cells called adipose tissue. Adiponectin is normally found in relatively high concentrations in healthy individuals. Its role in the body is to regulate the metabolism of lipids and glucose, which influences the body’s response to insulin and inflammation.
At Randox, our R&D Scientists are helping to change healthcare. By investing heavily into research and development to develop unique diagnostics tests, such as the adiponectin test, Randox provide doctors with the ability to identify disease risk sooner- offering the opportunity to prevent illness, rather than the need to find a cure.

Read the full Randox Reagents blog entry here
Randox Quality Control
One Simple Change to Randox Quality Control can save your laboratory time and money.
Randox Quality Control are a world leading manufacturer of true third party controls with over 390 analytes covering Antioxidants, Blood Gas, Cardiac Markers, Routine Chemistry, Coagulation, Haematology, Diabetes, Immunoassay, Immunology, Lipids, POCT, Therapeutic Drugs, Toxicology and Urine Chemistry, providing complete test menu consolidation. Randox Quality Control produces the most consistent material available with the most accurate target values.
Randox Quality Control guarantee to simplify QC practice in any laboratory, just ask one of their 60,000 users worldwide.
Find out more information about Randox Quality Control in the video above
Randox Toxicology
Randox Toxicology provides trusted solutions for the screening for drugs of abuse. With significant reinvestment in Research and Development, we persistently stay ahead of this ever challenging market. Being the first to develop New Psychoactive Substances tests such as fentanyl, bath salts and flakka allows us to maintain our position as a global leader.
Our pioneering technology has created a number of advancements in the field of toxicology. In particular, our patented Biochip Array Technology which can simultaneously screen from a multi-analyte testing platform, achieving a complete immunoassay profile from the initial screening phase.

Read the full Randox Toxicology blog post here
Randox Biosciences
During British Science Week, we are delighted to introduce you to our latest development utilising this technology; our Gastropanel Array,* a multiplex test engineered to diagnose those at risk of developing peptic ulcers and gastric cancer using non-invasive methods.
Our Gastropanel Array encompasses two quantitative assays, a H. pylori assay for the detection of antibodies produced in response to a H. pylori infection, a common cause of gastric cancer1 as well as a 3plex Gastropanel assay, for the detection of pepsinogen I (PGI), pepsinogen II (PGII) and gastrin 17 (G17).
Currently recorded as the world’s 5th most common cancer, the majority of gastric cancer cases are diagnosed after presenting as an emergency, when treatment may be less effective due to the cancer being at an advanced stage, highlighting the need for the availability of diagnostics tests like our Gastropanel Array to enable practitioners to administer prompt treatment and ultimately increase survival rates on a global scale.

Read the full Randox Biosciences blog here
Randox Testing Services
Randox Testing Services have shown how they are at the forefront of continually reacting and developing tests for NPS. NPS (formerley known as Legal Highs) have had devastating effects on users since emerging in the UK in 2008. These substances are highly dangerous and have caused unnecessary deaths. This is due to the effects from different elements used in production. Legislation concerning the substances changed in 2016 with the implementation of the Psychoactive Substance Act.
How have Randox Testing Services implemented change? Find out in the video above
Randox Food Diagnostics
Of the 41 antibiotics that are approved for use in food-producing animals by the FDA, 31 are medically important for human health. Randox Food Diagnostics provides advanced screening solutions for 94% of these antibiotics including beta-lactams, quinolones and tetracyclines, allowing you to ensure the integrity of your end product without compromising quality. Randox Food provides multiplex screening solutions validated across a range of matrices including urine, serum, tissue, milk, honey and feed.
The Evidence Investigator matched with Biochip Array Technology (BAT) provide the end user with fast, reliable results to aid in ensuring your produce is antibiotic free. BAT provides a platform for the simultaneous determination of multiple drug residues from a single sample using miniaturised immunoassays with implications in the reduction of sample/reagent consumption and an increase in the output of test results.

To read Randox Food Diagnostics full blog click here
We hope you enjoyed our informative British Science Week content from each of our Product Groups.
Look out for our Quiz later this week to test your knowledge on the #ScienceBehindRandox
Clin Lab 2017

CLIN LAB 2017
Randox Laboratories will be attending Clin Lab from 6th – 8th April 2017. We look forward to meeting you at Pragati Maidan Exhibition Center, India at Stand No. 9C08, Hall 9.
Randox Laboratories is a global leader in healthcare diagnostics. We are dedicated to developing solutions that not only meet your needs, but that are of the highest quality, the most reliable and the most cost-effective.
JOIN US FOR LIVE DEMONSTRATIONS
Drop by our stand to receive a live demonstration of our latest products including Acusera 24.7, RX imola and RX daytona+. These will take place at Stand No. 9C08, Hall 9 from Thursday – Saturday.
Acusera 24.7
Reduce time spent troubleshooting and analysing QC data with unique access to instantly updated peer group statistics and automatic calculation of Measurement Uncertainty, Sigma Metrics and Total Error all on one centralised platform.
RX series
The RX Series is globally renowned for quality and reliability, teamed with one of the most extensive dedicated clinical chemistry test menus on the market you can benefit from real cost savings through the consolidation of routine and specialised tests on a single platform.
ALSO SHOWCASING
RX series
The RX series of clinical chemistry analysers combines robust hardware and intuitive software ensuring unrivalled precision and accuracy for results you can trust.
Reagents
Committed to advancing biochemistry testing for laboratories. We offer a range of high quality reagents that cover routine and novel markers with applications for use on a wide range of clinical chemistry analysers.
Quality Control
Delivering accurate results, Randox QC provides the complete laboratory package combining true third party controls, calibration verification material, interlaboratory data management software and the world’s largest international EQA scheme – RIQAS.
What is Measurement of Uncertainty?
Measurement Uncertainty (MU) relates to the margin of doubt that exists for the result of any measurement, as well as how significant the doubt is. For example, a piece of string may measure 20 cm plus or minus 1 cm, at the 95% confidence level. As a result, this could be written: 20 cm ±1 cm, with a confidence of 95%. Therefore, we are 95% sure that the piece of string is between 19 cm and 21 cm long.
Standards such as ISO 15189 require that the laboratory must determine uncertainty for each test. However, they have not specified how this should be done.
How do we calculate Measurement Uncertainty using QC data?
Employing your QC data to calculate uncertainty makes several assumptions; your test system is under control, the patient samples are treated in the same manner as your controls and gross outliers have been removed. If you choose to use your QC data to calculate this you should ensure that you use a commutable control with a matrix similar to that of a patient sample, with analytes present at clinically relevant levels
To calculate MU, labs must look at the intra-assay precision and inter-assay precision of their test.
Intra-assay precision: Sometimes known as ‘within run’ precision, is where 20 or more replicates of the same sample are run at the same time, under the same conditions (calculated from a single experiment). Intra-assay precision helps to assess systematic uncertainties
Inter-assay precision: Sometimes known as ‘between run’ precision, is where 20 or more replicates are run at different times – e.g. 1 replicate every day for 20 days (can be calculated from routine IQC data). Inter-assay precision can help identify random uncertainties within the test system.
*The Australian Association of Clinical Biochemists (AACB) recommends that at least 6 months’ worth of QC data are used when calculating the inter-assay precision1.
Once the data is collected, you must calculate the standard error of the mean (SEM) of the intra-assay precision (A) and the SD of the inter-assay precision (B) in order to measure the uncertainty (u). Once A and B have been calculated, they need to be squared, added together and the square root of the sum found:
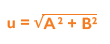
As uncertainty is calculated as SD and 1SD is equal to 68% confidence on a standard Gaussian curve, we can conclude that if we multiply using a coverage factor of 2, we can attain 2SD confidence of 95%. This is known as the Expanded Uncertainty (U):
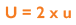
What is the Advantage of Measurement Uncertainty for a lab?
Labs need to carry out MU as it is a requirement of ISO 15189. It states: “The laboratory shall determine measurement uncertainty for each measurement procedure, in the examination phases used to report measured quantity values on patients’ samples. The laboratory shall define the performance requirements for the measurement uncertainty of each measurement procedure and regularly review estimates of measurement uncertainty”.
MU also helps determine whether the difference between two results is negligible due to uncertainty or significant due to a genuine change in condition of the patient; giving labs a greater confidence in reported results.
How can Randox help?
Our new Acusera 24.7 Live Online software provides automatic calculation of MU, saving valuable time and helping labs meet ISO 15189 requirements with ease.
Contact marketing@randox.com to find out how your lab can benefit from Acusera 24.7 Live Online
Fabricado en Europa – Randox Control de Calidad
Ubicados en Europa, la misión de Randox Control de Calidad es la producción de materiales de alta calidad que ayuden a racionalizar los procesos de control de calidad, además de ayudar a ahorrar tiempo y dinero a cualquier laboratorio, independientemente de su tamaño y presupuesto. Gracias a nuestra extensa oferta de productos, en los que se incluyen controles internos de tercera opinión, un software para la gestión de datos inter laboratorios y una amplísima variedad de Programas de Evaluación Externa de Calidad (PEEC), podrá obtener una visión completa del rendimiento de su laboratorio.
Al ser uno de los mayores fabricantes de controles auténticamente conmutables, ofrecemos soluciones para el control de calidad económicas, de la más alta calidad y diseñadas para la evaluación de los resultados de las pruebas de los pacientes. Con nuestra gama de controles Acusera podrá controlar lo que hasta ahora le supondría hasta 4 controles diferentes con un único vial Acusera, que junto con nuestra estabilidad sin igual y la elevada vida útil, le ayudarán a ahorrar tiempo y dinero. Además, las concentraciones son clínicamente relevantes, garantizando la precisión y fiabilidad de los instrumentos a lo largo de toda la variabilidad clínica.
Acusera 24.7 gestión de datos interlaboratorios
Acusera 24.7 le permitirá obtener resultados más precisos y reducir el tiempo que implica la supervisión e interpretación de los datos del control de calidad. Además, tendrá acceso a una gran variedad de funciones como a los datos del grupo par actualizados en tiempo real y el cálculo automático de la Incertidumbre, el Error Total y las Métricas Sigma. Con Acusera 24.7 podrá asegurar la calidad de sus análisis a través de la resolución más rápida de los problemas mediante esta plataforma centralizada.
RIQAS es el Programa de Evaluación Externa de Calidad (PEEC) de preferencia, empleado por más de 45.000 participantes en 124 países. Gracias a la frecuencia de los informes, podrá identificar los errores rápidamente, reduciendo la necesidad de realizar repeticiones innecesarias y costosas, haciendo que su laboratorio ahorre tiempo y recursos valiosos.
Take steps to prevent incorrect patient results by making one simple change
According to the NHS Litigation Authority; in 2015 within the UK alone, £193,680,744.30 was spent on ‘wrong diagnosis’ or ‘failed/delayed diagnosis’ causing huge financial strain and impact on labs.
With approximately 75% of clinical decisions and diagnosis based on laboratory test results. The only way to guarantee a high degree of accuracy is to implement a good Quality Control plan. The importance of this is recognised globally, several bodies exist internationally including ISO (International organisation for standardisation) who have developed a set of guidelines and quality systems to ensure the reliability of laboratory test results.
So what can you do to improve accuracy and reliability?
Choose a third party QC
ISO 151589:2012 Section 5.6.2.2 states that “the use of third party control materials should be considered, either instead of, or in addition to, any control materials supplied by the reagent or instrument manufacturer”.
First Party Controls are those manufactured by the instrument/reagent manufacturer. These controls are optimised specifically for use with the manufacturers test system and therefore will mask a multitude of weaknesses. First Party Controls tend to result in perceived accuracy and a biased assessment of performance.
Third Party Controls on the other hand are designed to be completely independent and are not optimised for use with a specific test or system. Leading manufacturers of third party controls will assign target values based on data collected from thousands of independent laboratories, ensuring the availability of statistically robust multi-method, multi-analyser data. Therefore laboratories using Third Party Controls can be assured of unbiased error detection across multiple platforms.
Randox Acusera is a world leading manufacturer of true third party controls providing a cost effective, high quality solution for any laboratory-regardless of size or budget.
Look out for QC samples with clinically relevant concentrations
ISO 15189:2012 states that ‘The laboratory should choose concentrations of control materials wherever possible, especially at or near clinical decision values, which ensure the validity of decisions made’.
It is important to assess the full clinical range of an assay i.e. the range between the lowest and highest results which can be reliably reported. In order to make sure a laboratory instrument is performing accurately across the full clinical range and in particular at the medical decision level, QC materials that cover low, normal and elevated concentrations should be used.
Due to the superior manufacturing process used by Randox, QC target values consistently cover the MDL of tests. By ensuring the controls in use cover clinical decision levels laboratories can be confident of the reliability and accuracy of the patient results they release.
Opt for a commutable control material
A good QC material has many essential properties but above all, controls must perform consistently and reflect the performance of patient samples – if a control meets these requirements then we can say it is commutable. Having a commutable control would aid in the prevention of incorrect patient results because they replicate the performance of a patient sample and react to the test system in a similar manner. Use of a commutable control will also reduce costly shifts in QC target values when reagent batch is changed.
At Randox we take quality seriously, that’s why all QC products are manufactured to the highest possible standard, delivering controls of unrivalled quality. Designed to be commutable, the Acusera range will ensure accurate and reliable instrument performance while simultaneously helping laboratories to meet ISO 15189:2012 requirements. A good QC process will include the use of Third Party Controls, Clinically Relevant Concentrations and controls which can be described as commutable. By employing Quality Control’s that encompass these traits, a laboratory professional can be certain that they have taken the necessary steps to decrease incorrect results and therefore potential misdiagnosis.
Acusera 24•7 Frequently Asked Questions (FAQ’s)

Is it possible to compare multiple instruments on a single Levey-Jennings Chart?
With Acusera 24•7 users can combine multiple instruments, as well as analytes and QC lots on a single chart. This will enable comparative performance assessment and facilitate immediate visualisation of any ongoing or emerging trends. It may also be useful for troubleshooting out of control QC events.
Is it possible to compare different levels of control on a single Levey-Jennings Chart for identification of concentration related bias?
Yes data for multiple QC lots can be displayed on a single, convenient Levey-Jennings chart allowing any concentration related problems to be identified quickly.
How often is peer group data updated?
With Acusera 24•7, peer group data is uniquely updated live in real-time. The instant nature of the peer data will help reduce time and money spent troubleshooting, re-running QC samples and performing any instrument maintenance. With real-time peer group data you can compare to other laboratories around the globe using the same lot of QC material and identify if there are any issues and whether they are unique to your lab or a widespread issue.
Is there a limit to the number of users?
There is no limit to the number of concurrent users – you can have as many as you want. There are 5 different levels of user access – Admin, Group Co-Ordinator, Manager, User and Technical Support. It is worth noting that User access can be customised per user to ensure access to only the required functionality
How can the software help me to meet ISO 15189:2012 requirements?
Unique to Acusera 24.7 , our software will automatically calculate Measurement Uncertainty (UM) and provide your laboratory with a printable report that can be used to help meet ISO 15189:2012 accreditation requirements. In addition to this the software can help to prevent the release of patient results in the event of a QC failure for example when the Quality Control rules are violated.
Is it possible to use RiliBAK as my allowable limits?
QC data can indeed be rejected or alerted based on RiliBAK guidelines. Other options are also available, including, CLIA, Biological Variation, RIQAS TDPA and user defined performance limits.
Is Acusera 24.7 secure?
To authenticate users, a number of security measures are used, including; participant number, username and a password combination (for individual role-based accounts). Password complexity standards are enforced on user account setup. CAPTCHA is enforced after several failed login attempts to prevent or guard against automated attacks. HTTPS and X509 certificate authentication is in place meeting industry security standards.
I have never used interlaboratory software before, is there training provided with my purchase of 247?
There are a number of different options available in terms of training. The easiest, most convenient and accessible form of training is through the use of our walk through demo that has voice-over and text so everyone can follow. With our software comes a user guide, this is a walk through that laboratories can use to guide them through the usage of the software. Additionally, there is also the possibility of a live demo from the sales consultant in your area who will be able to run through the software with you.
Can I create unique login credentials for each user?
Yes, each user will have their own personal log-in. If a lower level user, e.g. user, technical support or manager forgets their password they can have it reset by the administrator. However, if a group co-ordinator or admin forgets their password they should contact Randox directly who will be able to reset their credentials and resend to the administrator’s email address.
I currently use non-Randox Quality Controls, does this affect the ability of the software?
Not at all, our software is so flexible that it can be used with any other manufacturers Quality Control material. However, you will only have access to the internal functions of the software and will not have access to peer group statistics. For this reason we recommend the software is used alongside our Acusera true third party Quality Control solutions.
Is Acusera 24.7 the only software option available?
Yes this is the only option available – Unlike other manufacturers there is no need for any additional software packages or options. All functionality is available in the one software package.
My computer is very old, do I need a new, modern and up-to-date operating system to run the software?
Not necessarily. As long as you have stable access to the internet you can access Acusera 24.7 as it is a cloud based software.
My patient data is confidential. Will the software need access to this data?
Acusera 24.7 will not require access to your patient data. This is important for a laboratory but less for the software. Acusera 24.7 will only need access to the results of your Quality Control to ensure that your instrument/s are performing to standard and therefore ensuring that your patient results will be reliable and accurate.
I have forgotten my username and password – what do I do now?
If an individual with user level or manager level access forgets their username and password, they should contact the laboratory administrator. If an administrator or group co-ordinator forgets their username or password they should contact Randox who will verify the administrator and send new login details for the account.
Is Acusera 24.7 Connect required to import QC data?
Acusera 24.7 Connect is only required if you wish to import QC data automatically. Data can also be entered manually using the data entry screen or in a semi-automated manner using the EDI function.
I need to renew my license, is this done automatically?
If your licence has expired and you would like to renew you should get in touch with your local Sales Consultant.
My internet connection isn’t great. Will this affect the running of the software? What happens if the signal drops when entering results?
If connection is lost from the laboratory’s side, all data will be transferred to the web and once reconnected, the previous session will also be remembered. Emergency power generators and fall over servers are in place to ensure 99.8% uptime is guaranteed.
Are there any additional software requirements?
You must have access to a Java applet. This software is available as standard on almost all modern computers, laptops and notebooks.










